No CrossRef data available.
Article contents
Advances in diagnostic approaches to Fasciola infection in animals and humans: An overviews
Published online by Cambridge University Press: 25 January 2024
Abstract
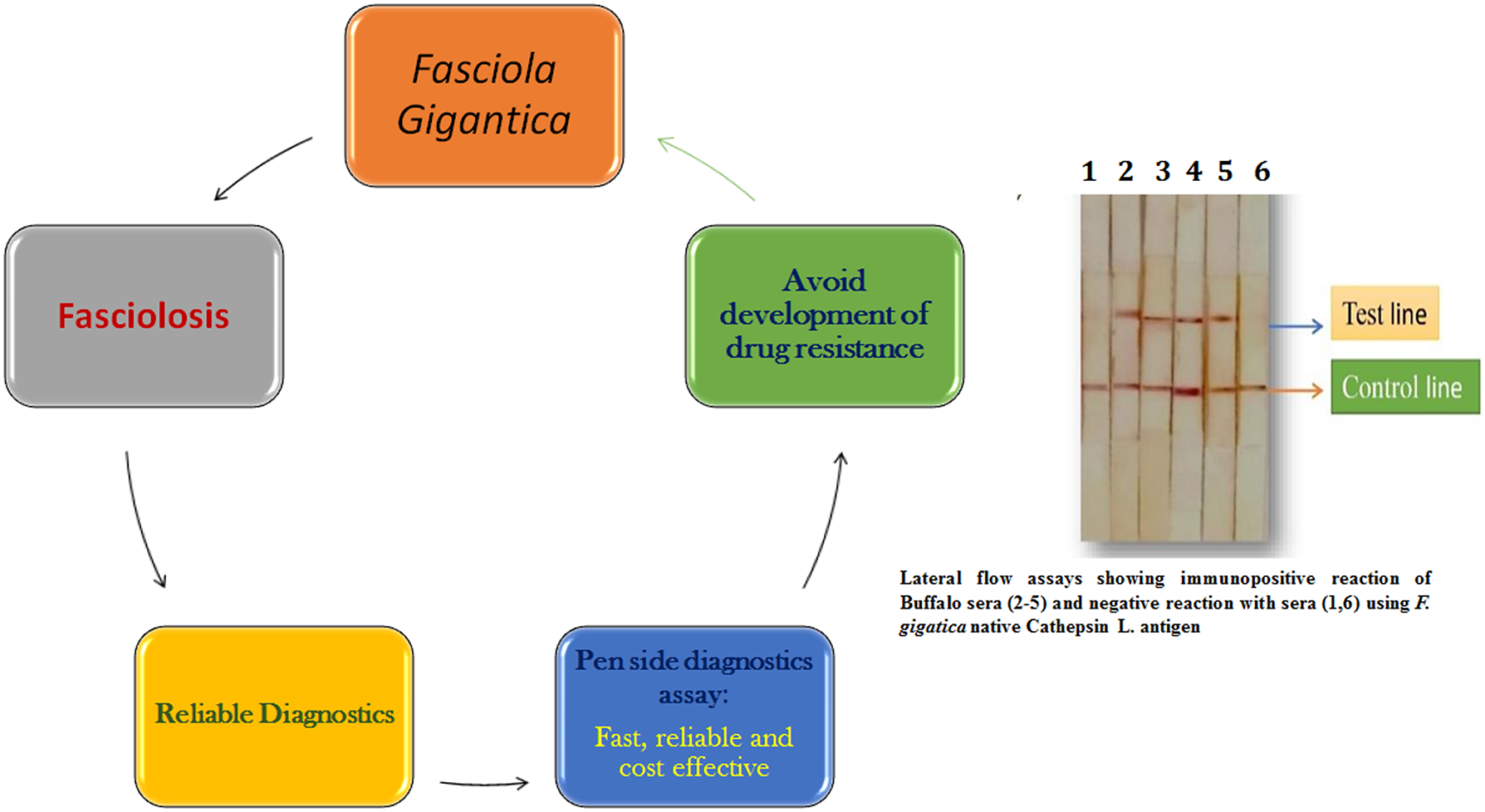
Fasciolosis, caused by Fasciola hepatica and F. gigantica, is an impediment to the livestock industry’s expansion and has a massively negative socio-economic impact due to its widespread prevalence in livestock. It is a waterborne zoonosis affecting human populations in the countries where rural economies are associated with livestock rearing. Conventional diagnosis of Fasciola infection is done by detecting parasite eggs in the faeces of infected animals or by immunological methods. Accurate and quick immunodiagnosis of Fasciola infection in animals and humans is based on the detection of antibodies and specific antigens expressed in the prepatent stage of the parasite. Both molecular and serodiagnostic tests developed thus far have enhanced the reliability of Fasciola diagnosis in both man and animals but are not widely available in resource-poor nations. A pen-side diagnostic test based on a lateral flow assay or a DNA test like loop-mediated isothermal amplification (LAMP) would be simple, fast, and cost-effective, enabling clinicians to treat animals in a targeted manner and avoid the development of drug resistance to the limited flukicides. This review focuses on the recent advances made in the diagnosis of this parasite infection in animals and humans.
- Type
- Review Article
- Information
- Copyright
- © The Author(s), 2024. Published by Cambridge University Press




