Chronic inflammatory bowel disease (IBD), mainly ulcerative colitis and Crohn's disease, is a naturally remitting and recurring condition of the digestive tract. Although its precise aetiology is unknown, it is probably related to an abnormal exacerbated immune response to otherwise innocuous stimuli, which is not properly abrogated by the feedback system that normally down-regulates the mucosal response to luminal factors(Reference Fiocchi1). As in other inflammatory processes, IBD is characterised by an up-regulation of the synthesis and release of a variety of pro-inflammatory mediators, such as eicosanoids, platelet-activating factor, reactive oxygen and N metabolites and cytokines, thus influencing mucosal integrity and leading to excessive tissue injury(Reference Katz, Itoh and Fiocchi2, Reference Podolsk, Fiocchi and Kirsner3). Moreover, most of these mediators can induce the biosynthesis and release of other such compounds, generating a ‘vicious cycle’ that may result in the propagation and perpetuation of the inflammatory response.
Various pro-inflammatory cytokines, such as IL-1, IL-6 and TNF-α, chemokines and adhesion molecules are known to contribute to IBD pathogenesis(Reference Sandborn and Yednock4, Reference Kurtovic and Segal5). In particular, TNF-α plays a crucial activating role among the cytokines that modulate endothelial functions. In fact, TNF-α regulates the expression of some adhesion molecules and cytokines in endothelial cells and also increases vascular permeability(Reference Sasaki, Elrod and Jordan6).
Inhibition of NF-κB activation has been suggested as an anti-inflammatory treatment strategy for IBD. The nuclear transcription factor NF-κB is a key regulator of the inducible expression of many genes involved in immune and inflammatory responses in the gut. Stimuli such as oxidative stress, cytokines (IL-1, IL-6 and TNF-α), bacteria and viruses can release NF-κB to allow translocation to the nucleus(Reference Dijkstra, Moshage and Jansen7).
During the last decades, it has become increasingly clear that NO overproduction by inducible NO synthase (iNOS) is deleterious to intestinal function(Reference Hogaboam, Jacobson and Collins8–Reference Salas, Gironella and Soriano10), thus contributing significantly to gastrointestinal immunopathology during the chronic inflammatory events that take place in IBD(Reference Gricontrol, Pavlick and Laroux11).
Conventional colitis therapies can reduce periods of active disease and help preserve remission. However, these treatments often bring marginal results, and the disease can become refractory(Reference Larrosa, Tome-Carneiro and Yanez-Gascon12, Reference Singh, Singh and Singh13). In addition, presently, no definitive surgical cure exists for IBD(Reference Engel and Neurath14). As a result of the lack of efficacious conventional treatments, up to 40 % of IBD patients are estimated to use some form of megavitamin or herbal or even dietary supplement to complement conventional therapies(Reference Singh, Singh and Singh13, Reference Head and Jurenka15, Reference Head and Jurenka16).
Flavonoids comprise a large group of compounds occurring widely throughout the plant kingdom. Daily polyphenol intake (present in apple, grape, wine and herbs) in the human diet is highly variable, with estimations ranging from 23 mg/d to more than 500 mg/d. Polyphenols exert several biological activities mainly related to their ability to inhibit enzymes, act as antioxidants and regulate the immune response(Reference Middleton, Kandaswami and Theoharides17).
Grapes and their derived products are considered major sources of phenolic compounds, categorised as flavonoids and non-flavonoids(Reference Jarowski and Lee18). The first group comprises catechins, epicatechins, epigallocatechin, kaempferol, quercetin, myricetin and anthocyanins among others. The second group includes phenolic acids, hydroxybenzoic and hydroxycinnamic acids and stilbenes, the subgroup that contains the resveratrol molecule(Reference Lee and Jarowski19–Reference Auw, Blanco and O'Keefe21). This phytoalexin is synthesised in the skin of the fruit as a response to stress caused by fungal attack (Botrytis cinerea and Plasmopara viticola), mechanical damage or UV light irradiation(Reference Sautter, Denardin and Alves22). Thus, the goal of the present study was to evaluate the mechanisms of action of phenolic compounds present in grape juice concentrate on 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis.
Materials and methods
General procedures
All experimental protocols involving animals conformed to the procedures described in the Guiding Principles for the Use of Laboratory Animals, and the study was approved by the Animal Committee of the Universidade Federal de São Paulo, UNIFESP.
A total of forty-one adult male Wistar rats (Rattus norvegicus albinus), weighing 250–300 g, provided by the Center for the Development of Experimental Models for Medicine and Biology of the Universidade Federal de São Paulo, were maintained in restricted-access rooms at a controlled temperature (23°C) and on a 12 h light–12 h dark cycle. Standard laboratory chow and drinking water were provided ad libitum.
Before initiating the experimental procedures, the animals were treated with Panacur® to eliminate intestinal parasites; two doses were administered orally with an interval of 3–5 d before the experiment.
Induction of colitis and treatment protocols
Colitis was induced by TNBS using the modified method described by Morris et al. (Reference Morris, Beck and Herridge23). Briefly, rats were subjected to fasting 1 d before the experiment. Each rat was anaesthetised with ketamine and xylazine and colitis was induced by the administration of 50 mg/kg of TNBS (Sigma) in ethanol (50 %, v/v) in a total volume of 1 ml, via a rubber catheter inserted 8 cm into the colon from the anus. The control group received 1 ml of 0·9 % saline solution by the same technique.
The animals were randomised into seven groups in a blinded fashion, as follows: negative control group, non-treated animals; TNBS group, non-treated induced colitis; 2 % grape juice concentrate control group; 1 % grape juice concentrate 24 h after TNBS colitis induction; 1 % grape juice concentrate on day 7 after colitis induction; 2 % grape juice concentrate 24 h after colitis induction; 2 % grape juice concentrate on day 7 after colitis induction.
The animals were given grape juice (G8000™) supplied by Golden Sucos in their drinking water (at concentrations of 1 and 2 %), 24 h or on day 7 after colitis induction or on the same day in the control group. The dose at 1 % was calculated on the basis of amount of polyphenols to be equivalent to four glasses (200 ml each) of natural grape juice and adjusted to animal metabolism (twice as fast as humans)(Reference Gollucke, Souza and Tavares24). According to the American Dietetic Association, human consumption of approximately 200–500 ml presents moderate to strong evidence of a positive physiological effect such as platelet aggregation(25). The chemical characterisation of grape juice concentrate was performed in a previous study conducted by our research group(Reference Kim, Lee and Lee26). The following compounds were identified: dimethoxy-flavylium, disaccharide, fatty acids, palmitic and linoleic acids. Caffeoyltartaric, fertaric and caffeoylquinic acids appeared as the main organic acids identified. Quercetin appeared as a single molecule and in the glucoronide form. Resveratrol was also identified in relatively significant abundance.
All animals were checked daily for behaviour and general health conditions and body mass was recorded weekly.
Determination of total phenols and in vitro antioxidant activity of grape juice concentrate
Total phenols were measured by the Folin–Ciocalteu assay(Reference Singleton and Rossi27) using gallic acid (Sigma-Aldrich) for the standard curve and the results expressed as mg gallic acid equivalents/kg. The readings (in triplicate) were taken at 740 nm using a Genesis 2 spectrometer (Thermo-scientific). For the evaluation of in vitro antioxidant activity, the 1,1-diphenyl-2-picrylhydrazyl (Sigma-Aldrich) assay was used based on the methods of Brand-Williams et al. (Reference Brand-Williams, Cuvelier and Berset28) as modified by Kim et al. (Reference Kim, Lee and Lee26). Absorbance was measured with a Beckman spectrometer at 517 nm before the addition of samples and after 30 min; the difference was plotted on a vitamin C (ascorbic acid; Merck) standard curve. Analyses were carried out in triplicate and the results expressed in mg vitamin C equivalent antioxidant capacity/kg. The degree of confidence for this method is 99 %.
Morphological evaluation of colon lesions
All rats were killed on day 16 following the experiment. The colon was removed from the caecum to the anus and opened by a longitudinal incision. The tissue was rinsed with saline and tissue damage was examined. The criteria of the macroscopic score used a previously validated scoring system (Table 1)(Reference Peran, Camuesco and Comalada29).
Table 1 Criteria for the assessment of macroscopic colonic damage (modified from Peran et al. (Reference Peran, Camuesco and Comalada29))

After macroscopic assessment of the mucosa, samples were fixed in 10 % buffered formalin, embedded in paraffin and tissue sections were stained with haematoxylin and eosin for histopathological evaluation of colonic damage by light microscopy. The assessment criteria of the histological score was according to previous literature (Table 2)(Reference Appleyard and Wallace30).
Table 2 Criteria for the assessment of microscopic colonic damage (modified from Appleyard & Wallace(Reference Appleyard and Wallace30))

Preparation of total RNA
Frozen colonic tissue at − 86°C was homogenised and total RNA was isolated using cold Trizol Reagent (Invitrogen), according to the manufacturer's instructions. Total RNA was determined using a NanoDrop® ND-1000 spectrophotometer (NanoDrop Technologies). RNA samples were treated with DNase (Invitrogen) to avoid contamination with genomic DNA.
Complementary DNA synthesis and quantitative real-time PCR
Complementary DNA synthesis was performed using RT M-MVL (Invitrogen), according to the manufacturer's instructions. Real-time PCR was performed in an ABI Prism 17 500 Real-Time PCR system using the SYBR Green Kit (Applied Biosystems).
Primers for the specific amplification of each complementary DNA were designed using Primer Express software (Applied Biosystems), considering established criteria, such as product length, optimal PCR annealing temperature and the likelihood of primer self-annealing. The following primers were used for each gene: TNF-α, forward 5′-CCC AGA AAA GCA AGC AAC CA-3′ and reverse 5′-GCC TCG GGC CAG TGT ATG-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH), forward 5′-GCT CTC TGC TCC TCC CTG TTC-3′ and reverse 5′-GAC GCT GGC ACT GCA CAA-3′; NF-κB, forward 5′-AAG ATC AAT GGC TAC ACG GG-3′ and reverse 5′-CCT CAA TGT CTT CTT TCT GC-3′; iNOS, forward 5′-CAG TGG CAA CAT CAG GTC-3′ and reverse 5′-GGT CTC GCA CTC CAA TCT-3′; intercellular adhesion molecule-1 (ICAM-1), forward 5′-CAC TAG AGG AGT GAG CAG GTT AAC AT-3′ and reverse 5′-TAT GAC TCG TGA AAG AAA TCA GCT CTT-3′. glyceraldehyde 3-phosphate dehydrogenase (GADPH), forward 5′-CTA CCC ACG GCA AGT TCA AAC-3′ and reverse 5′-CCA GTA GAC TCC ACG ACA TAC-3′ (included in the technique as the housekeeping gene; internal control).
PCR were performed in triplicate containing 12 μl of final volume using 3·0 μl of a 1:5 (v/v) dilution of complementary DNA, 3·0 μl of primer mix (600 nm, forward and reverse) and 6·0 μl of SYBR Green master mix 2 × (Applied Biosystems). The reactions were performed in MicroAmp ninety-six-well plates (Applied Biosystems) covered with optical adhesive (Applied Biosystems). Samples were subjected to forty cycles of 95°C for 10 min, 95°C for 15 s and 60°C for 1 min. An amplification efficiency curve using different complementary DNA dilutions was also performed for each gene tested.
To normalise the data for the control and experimental groups, arbitrary units were calculated as: arbitrary unit = 2− ΔΔC T and ΔΔC T= sample ΔC T− control ΔC T, where C T is the threshold cycle.
Statistical analysis
Results are expressed as means with their standard errors. All data were converted into logarithmic transformation to achieve normal distribution. Statistical analysis was performed by one-way ANOVA followed by Tukey's test using GraphPad Prism software version 4.0 (GraphPad Software, Inc.). A value of P< 0·05 was considered for statistical significance.
Results
Clinical evaluation
All rats exposed to TNBS showed a significant decrease in body mass from day 1 to day 5 after colitis induction, with subsequent recovery (Fig. 1). The animals developed severe diarrhoea on day 2 after colitis induction and rectal bleeding was occasionally observed. Nevertheless, the 1 % grape juice-treated induced colitis group showed marked clinical improvement when compared with the TNBS-induced colitis group. In the negative control and 2 % grape juice control groups, no alterations were observed (P>0·05).

Fig. 1 Body mass alterations in the different groups. ![]() , Control group, non-treated animals;
, Control group, non-treated animals; ![]() , 2,4,6-trinitrobenzene sulphonic acid (TNBS) group, non-treated induced colitis;
, 2,4,6-trinitrobenzene sulphonic acid (TNBS) group, non-treated induced colitis; ![]() , 2 % grape juice control group;
, 2 % grape juice control group; ![]() , 1 % grape juice 24 h after TNBS colitis induction;
, 1 % grape juice 24 h after TNBS colitis induction; ![]() , 1 % grape juice on day 7 after colitis induction;
, 1 % grape juice on day 7 after colitis induction; ![]() , 2 % grape juice 24 h after colitis induction;
, 2 % grape juice 24 h after colitis induction; ![]() , 2 % grape juice on day 7 after colitis induction (A colour version of this figure can be found online at
http://www.journals.cambridge.org/bjn).
, 2 % grape juice on day 7 after colitis induction (A colour version of this figure can be found online at
http://www.journals.cambridge.org/bjn).
Macroscopic inspection of the caecum, colon and rectum showed a flacid appearance and evidence of bowel wall thickening, inflammation and ulcers (Fig. 2). The TNBS-induced colitis and 2 % grape juice-treated induced colitis groups showed higher tissue damage in the distal colon (7·83 (se 1·24) and 8·00 (se 1·78), respectively). The majority of the rats in this group showed greater thickening of the colon. However, the rats that received the grape juice at the 1 % dose on day 7 after colitis induction presented reduced intensity of the macroscopic score, when compared with rats treated with TNBS-induced colitis. In the negative and 2 % grape juice control groups, no macroscopic damage was observed (Table 3).
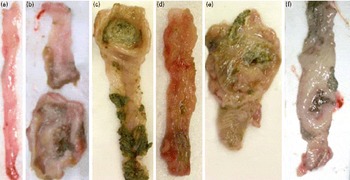
Fig. 2 Macroscopic observations of the colon. (a) 2 % Grape juice control group – intact colon; (b) 2,4,6-trinitrobenzene sulphonic acid (TNBS) group, non-treated induced colitis – severe thickening, ulcer and necrosis; (c) 1 % grape juice 24 h after TNBS colitis induction – less intense lesion; (d) 1 % grape juice on day 7 after colitis induction – mild lesion; (e) 2 % grape juice 24 h after colitis induction – severe shortening and thickening, ulcer and necrosis; (f) 2 % grape juice on day 7 after colitis induction – moderate lesion, colon less thickening (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
Table 3 Macroscopic and histological results following experimental colitis and treatment with grape juice concentrate (Mean values with their standard errors)

TNBS, 2,4,6-trinitrobenzene sulphonic acid.
* Mean value was significantly different when compared with the TNBS group (P< 0·05).
Histological analysis
On histological examination of the colon from the negative and 2 % grape juice control groups, the histological features of the colon were typical of a normal structure. On the other hand, the non-treated TNBS-induced colitis group presented inflammation extended through the mucosa, muscularis mucosae and submucosa (Fig. 3). The mucosa adjacent to ulcers showed crypt distortion and necrotic tissue. Extensive granulation tissue with the presence of fibroblasts and inflammatory cell infiltrate was also apparent (Fig. 3).

Fig. 3 Photomicrograph of the haematoxylin and eosin-stained section of rat colons in the different groups. (a) Control group and (b) 2 % grape juice control group – absence of morphological alterations; (c) 2,4,6-trinitrobenzene sulphonic acid (TNBS) control group; (d) 1 % grape juice 24 h after TNBS colitis induction; (e) 1 % grape juice on day 7 after colitis induction; (f) 2 % grape juice 24 h after colitis induction and (g) 2 % grape juice on day 7 after colitis induction – presence of transmural inflammation, ulceration, intense cellular inflammatory infiltrate (granulocytic and mononuclear) and distortion of the cellular architecture. Haematoxylin and eosin stain, 40 × magnification (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
Rats receiving grape juice at the 1 % dose on day 7 after colitis induction presented an attenuation of morphological signs of cell damage. In this group, statistically significant differences (P< 0·05) occurred in the histological analysis when compared with the TNBS group only (Table 3). The TNBS-induced colitis and 2 % grape juice-treated induced colitis groups showed higher tissue damage in the distal colon when compared with the respective controls (11·67 (se 0·33); 13·25 (se 0·47), respectively).
Analysis of TNF-α, inducible nitric oxide synthase, intercellular adhesion molecule-1 and NF-κB mRNA expression by quantitative RT-PCR
The results of the total phenol content of grape juice concentrate showed a concentration of 45·8 g gallic acid equivalents/kg with an antioxidant activity of 27·3 g vitamin C equivalent antioxidant capacity/kg.
Table 4 shows the results from the gene expression of TNBS inducing colitis and treated with grape juice. Statistically significant differences (P< 0·05) in TNF-α mRNA expression were detected in the TNBS group. However, the treatment with grape juice at the 1 % dose after 7 d of inducing colitis was able to decrease the TNF-α expression.
Table 4 Analysis of mRNA expression in experimental colitis and treatment with grape juice concentrate (Mean values with their standard errors)

iNOS, inducible NO synthase; ICAM-1, intercellular adhesion molecule-1; TNBS, 2,4,6-trinitrobenzene sulphonic acid.
* Mean value was significantly different when compared with the TNBS group (P< 0·05).
Regarding iNOS expression, remarkable changes were noted. The treatment with grape juice for both doses 7 d after inducing colitis was able to decrease the iNOS expression when compared with the TNBS group. With respect to NF-κB, the administration of grape juice did not show significant results among the groups (Table 4).
ICAM-1 mRNA expression showed statistically significant differences (P< 0·05) in the TNBS group when compared with the negative control. Nevertheless, the treatment with grape juice did not show significant changes, regardless of the dose adopted in the present study (Table 4).
Discussion
Epidemiological studies have revealed that phenolic-rich diets reduce mortality induced by degenerative diseases caused by oxidative stress(Reference Scalbert, Johnson and Saltmarsh31). Among the various classes of phenolic compounds, flavonoids exert physiological properties that may be the source of health benefits from wine consumption(Reference Gürbüz, Göçmen and Dagdelen32). In grape juices, flavonoids are mostly found in the monomeric form with large differences among cultivars(Reference Jarowski and Lee18–Reference Auw, Blanco and O'Keefe21).
In the present study, we evaluate the mechanisms of action of phenolic compounds present in grape juice on TNBS-induced colitis. This model is accompanied by a marked thickening of the colon wall, infiltration of polymorphonuclear leucocytes and ulceration, resembling human Crohn's disease. The present results demonstrate that the administration of concentrated grape juice at the 1 % dose was able to reduce the severity and extension of damage caused by TNBS. It has been reported that proanthocyanidins from grape seeds were able to induce the recovery of the colon after the induction of recurrent colitis. The effect was demonstrated by reduced colonic weight:length ratio and macroscopic and microscopic damage scores(Reference Wang, Yang and Wang33).
Colonic injury induced by TNBS administration was also characterised by an increase in the pro-inflammatory cytokine TNF-α. In contrast, the levels of this cytokine were significantly lower (P< 0·05) in rats treated with grape juice at the 1 % dose on day 7. The effect was not observed in animals consuming the higher grape juice dose (2 %). In fact, earlier studies have demonstrated that higher doses of grape juice consumption are not able to exert some chemopreventive properties. For example, Jung et al. (Reference Jung, Wallig and Singletary34) have postulated that at the 3460 mg/l concentration of grape phenolics, the anti-dihydrodiolepoxide-dimethylberz[a]anthracere (DMBA)-dG adduct decreased significantly in the rat liver. However, no significant difference in animal growth was observed among the treatment groups throughout the tumour study. Consumption of grape juice was not associated with a significant change in mammary tumour incidence compared with rats fed the control fluid, nor were the numbers of palpable mammary tumours per rat and adenocarcinomas per rat significantly inhibited in a dose-dependent manner following administration of grape juice(Reference Jung, Wallig and Singletary34). Altogether, it seems that grape juice at 1 % was able to exert beneficial outcomes against experimental colitis in rats. Since animals suffering from experimental colitis and treated with grape juice at 2 % showed higher tissue damage in the distal colon when compared with the respective controls, we assumed that the dose was toxic. However, further studies are necessary to elucidate this issue.
The results obtained by Martin et al. (Reference Martin, Villegas and Sanchez-Hidalgo35), with a similar experimental protocol, showed that administration of resveratrol exerted a protective effect on TNBS-induced colitis. This could be verified by the absence of high regulation of TNF-α and the decrease in the expression of NF-κB considered a key regulator in inducing the expression of many genes involved in immune response and IBD(Reference Martin, Villegas and Sanchez-Hidalgo35). Proanthocyanidins from grape seeds also significantly reduced the expression levels of TNF-α, as well as the translocation of NF-κB in colonic mucosa after 7 d following colitis was twice induced by TNBS(Reference Wang, Ge and Yang36).
It is well documented that the NF-κB pathway can be inhibited by a variety of structurally diverse antioxidants, such as polyphenols. The molecular mechanisms involved in the suppressive effects of flavonoids on NF-κB are currently unknown, but several possibilities have been postulated. The first possibility is that flavonoids may inhibit NF-κB by acting as antioxidants, since NF-κB is a redox-sensitive transcription factor and activated by oxidant stress in the inflamed intestinal mucosa. The second possibility is that flavonoids inhibit NF-κB via blocking the phosphorylation as well as degradation of the inhibitor of κB protein, a crucial step in the translocation of NF-κB to the nucleus and its subsequent activation(Reference Bors, Heller and Michel37–Reference Rogler, Brand and Vogl39). In the present study, the effect of grape juice in the expression of NF-κB was not observed. Equally, no significant decrease in the expression of ICAM-1 was seen.
During the last decades, it has become increasingly clear that NO overproduction by iNOS is deleterious to intestinal function, thus contributing significantly to gastrointestinal immunopathology during the chronic inflammatory events that take place in IBD(Reference Hogaboam, Jacobson and Collins8–Reference Gricontrol, Pavlick and Laroux11). The important role attributed to NO in these intestinal conditions encouraged us to study the potential protective effects of grape juice phenolics on TNBS-induced colitis. The present findings demonstrated that the grape juice for both doses administered on day 7 after colitis induction modulated the iNOS expression. Such findings are fully in line with others(Reference Wang, Yang and Wang33).
To exclude the possibility that grape juice could bind TNBS and thus interferes with colitis development, an experiment was performed in which rats were treated daily by oral administration of grape juice at 2 %. It was observed that these animals showed no intestinal damage or abnormal expression of the genes in question. However, the administration of grape juice at a 2 % dose was toxic in rats with induced colitis. In fact, some studies have reported that not all forms of polyphenols have anti-inflammatory properties in vivo. Quercetin showed no beneficial effect on intestinal inflammation in vivo, despite being considered a flavonoid with antioxidant properties. However, its protective effect was observed for the inhibition of the NF-κB pathway in vitro studies.
Another study, conducted by Skyberg et al. (Reference Skyberg, Robison and Golden40), demonstrated the toxicity of high doses of polyphenols extracted from apple, in the treatment of colitis induced by dextran sodium sulphate. In this study, it was observed that mice that received the oral treatment showed improvement in the lesions; however, the intraperitoneal treatment was highly toxic to animals. Morimoto et al. (Reference Morimoto, Watanable and Yamori41) and Sakai et al. (Reference Sakai, Furoku and Nakamoto42) reported the toxicity of some forms of soyabean isoflavone extract on dextran sodium sulphate-induced colitis. Sakai et al. (Reference Sakai, Furoku and Nakamoto42) demonstrated that the mice treated with daidzein and especially those treated with equol showed a significant reduction in body weight. In addition, the survival of the equol-treated group was significantly reduced.
In summary, the present results suggest that the grape juice concentrate reduced the noxious effects induced by colitis caused by TNBS, especially at the 1 % dose. However, this suggestion should be verified by further investigation.
Acknowledgements
The present study was supported by CNPq and CAPES. The authors wish to express their gratitude to Professor Dr Nora Manoukian Forones (Division of Gastroenterology, Universidade Federal de São Paulo, Escola Paulista de Medicina, UNIFESP, SP, Brazil) and Joaquim Soares de Almeida (Department of Pathology, Universidade Federal de São Paulo, Escola Paulista de Medicina, UNIFESP, SP, Brazil) for technical assistance. D. A. R. is a recipient of a CNPq fellowship. A. P. R. P., A. P. B. G., S. J. M. and D. A. R. designed the study. A. P. R. P., V. L. P., P. M., R. M. S. and M. M. P. contributed to the experimental design. All authors interpreted the data and wrote the manuscript. The authors declare no conflict of interest.











