Status epilepticus (SE) is the most common neurological emergency in childhood, with a risk of long-term neurological sequelae.Reference DeLorenzo, Hauser and Towne 1 - Reference Hussain, Appleton and Thorburn 3 Status epilepticus has been defined as a single epileptic seizure >30 minutes (min) in duration or two or more sequential seizures during which function is not regained. 4 In clinical practice, however, shorter temporal criteria for generalized convulsive SE in adults and older children (more than five years of age) have been suggested.Reference Lowenstein, Bleck and Macdonald 5 , Reference Meldrum 6 Currently, the most common thresholds for considering prolonged seizures as SE are 5 and 30 minutes.Reference Sánchez Fernández, Abend and Agadi 7
Epidemiological studies identify the incidence of SE to be the highest for children under the age of one yearReference DeLorenzo, Hauser and Towne 1 , Reference Hesdorffer, Logroscino and Cascino 8 and ranging from 10-27/100,000.Reference Sánchez Fernández, Abend and Agadi 7 Status epilepticus etiology is poorly understood and tends to be a multifactorial condition, although the most common cause is infection.Reference Sánchez Fernández, Abend and Agadi 7 A population-based prospective cohort study from Finland suggests that more than one quarter of children with newly diagnosed epilepsy will experience SE, and nearly half of patients with one attack will experience a subsequent attack. The risk of SE has been deemed to be highest early in the course of paediatric epilepsy. Status epilepticus occurred prior to onset of epilepsy in over 73% of cases, and within two years of onset in 90% of cases.Reference Sillanpaa and Shinnar 9 Status epilepticus associated with epilepsy is approximately 15%, according to one observational study.Reference Sutter, Marsch and Fuhr 10 A substantial proportion (16%) of children with first ever-convulsive status epilepticus will have a recurrence within a year. Those with a previous neurological abnormality are more likely to have a recurrence.Reference Chin, Neville and Peckham 11 Risk factors that are strongly associated with SE recurrence include age at time of onset, remote symptomatic etiology, and epilepsy syndrome.Reference Shinnar, Pellock and Moshé 12 Overall, prognosis is dependent on the underlying etiology and seizure persistence.Reference Abend and Loddenkemper 13
Systematic studies on seizure duration in children have been few. On analysis of seizure duration in paediatric patients from ambulance and emergency room records, Shinnar, et al. found that while the mean seizure duration was approximately 12 minutes, 12% of cases presented with seizures lasting >30 minutes. Overall, the data suggested that if the seizure lasted for more than seven minutes there is 95% probability that it can be categorized with seizures that last for more than 30 minutes.Reference Shinnar, Berg and Moshe 14
Status Epilepticus outcomes from paediatric intensive care unit (PICU) accounting for variables such as transportation times, emergency department (ED) intervention, and intensive care unit (ICU) management have not yet been described. Children in SE who are admitted to the PICU are likely to represent the severe end of the spectrum of outcomes, which may reflect either the underlying cause, the treatment of SE, or both.Reference Wilkes and Tasker 15 While studies have described the mortality associated with SE, no studies have described the ICU-related morbidity and outcome of patients with SE. Overall mortality due to SE has declined over the past two decades because of more aggressive treatment occurring outside hospitals and stricter hospital management with clear, time-defined protocols.Reference Wilkes and Tasker 15 Nonetheless, prolonged SE has the potential for ICU-related morbidity, including increased duration of mechanical ventilation, hemodynamic instability, and multi-organ dysfunction.Reference Abend, Gutierrez-Colina and Dlugos 16 There are no published studies at this time evaluating SE and long-term neuropsychological outcomes.
Patient transportation and ED management can further affect SE outcomes due to temporal delays. Transport times for critically ill children in SE to tertiary care centers across Canada can be variable, with potentially longer transport times in prairie provinces. The purpose of this study was to explore the possible role of etiology of SE during the ICU course, and its effect on short-term neurological outcome of children admitted with SE to a tertiary care Canadian prairie PICU. We hypothesize that SE associated with prolonged transport times and PICU stay will lead to poor prognoses, including long-term neurological outcomes and increased morbidity. Data from PICU admissions for SE may clarify some of these issues and potentially facilitate the development of strategies to decrease the duration of SE and its complications.
Methods
We completed a retrospective audit of all PICU admissions at the Children’s Hospital of Winnipeg over a 10-year period (1994-2004). Institutional Review Board approval was obtained for the study. Patients under 18 years of age admitted to the PICU at the Children’s Hospital of Winnipeg, Manitoba with prolonged (>30 min) convulsive seizures were identified using a PICU-maintained database. Any patient with a diagnosis of SE that required admission to the PICU was included in the study, with the exception of those children with prolonged febrile seizures.
Demographic data, including age, weight, gender, and postal code were collected. The postal code was used to determine the location of a patient’s residence (within Winnipeg or outside the city) for transport information. Patients from Winnipeg were defined as Group A and patients from outside Winnipeg were labeled as Group B. Data were collected on treatment administered prior to transport to the Children’s Hospital, including treatment at home or within the community, at a nursing station, or at a community hospital. In addition, pre-PICU admission treatment details were documented, including anti-epileptic drugs (AEDs) that were administered, time of administration, and doses.
Transport data, as available, beginning from the initial site of SE management, were recorded as follows: time required for transport to Children’s Hospital, and the time of administration, doses and names of the AEDs given. Apnea, failed intubations, seizure evolution to SE, and refractory SE (defined as SE that did not respond to initial treatment with a first-line AED, such as benzodiazepine, plus any additional AED) were documented during transport, in the ED, and in the PICU. Durations of PICU and hospital stay were recorded. Seizure duration was calculated from hospital records. In addition, information about recurrent seizures was noted, including number of events and response to treatment. Complications of SE assessed in the PICU included: sepsis, renal failure, hyperthermia, cardiac arrhythmias, hypotension, pulmonary edema, hypoglycemia, cerebral edema, rhabdomyolysis, hepatic failure, disseminated intravascular coagulation, apnea, anemia, syndrome of inappropriate anti-diuretic hormone (SIADH), hypertension, and respiratory failure. Details of therapeutic coma in PICU were recorded, including drugs administered, duration of coma, duration of mechanical ventilation, and complications occurring secondary to mechanical ventilation. Outcome-related information recorded on patient discharge was divided into two categories based on whether the patient returned to baseline or developed new neurological deficits. New neurological deficits ranged from parental descriptions of behavioral deviations from pre-SE baseline to new abnormal neurological examination findings or new cognitive difficulties. The total number of deaths was also recorded.
Statistical analysis was performed using Student’s t-test for normal continuous variables. Medians and interquartile ranges were reported for highly skewed variables related to duration (transport times, seizure duration, time to first AED, time in the ED, length of PICU stay, therapeutic coma duration, mechanical ventilation duration, length of hospital stay). Non-parametric analysis using the Kruskal-Wallis test was used for outcome variables and postal code variability. The Chi-square test and Fisher’s exact test were used to analyze proportional variables, including transport complications, ED and PICU complications, and imaging rates for computed tomography (CT), magnetic resonance imaging (MRI), and electroencephalography (EEG). A p-value of <0.05 was considered significant for all analyses.
Results
Of 230 patient charts reviewed, 189 patients met study criteria. Forty patients were excluded because of febrile seizures lasting less than 30 minutes, a seizure diagnosis was ruled out, or the chart data were incomplete. Forty patients were excluded due to febrile seizures lasting less than 30 minutes, a seizure diagnosis was ruled out, or the chart data were incomplete.
Demographics
The median age of the patients was 1.88 years (interquartile range 0.83-5.00). These children had a median weight of 12.00 kg (interquartile range 8.68-20.00). Of the 189 patients, 103 (54.21%) were male, and 87 (45.79%) were female.
Etiology
Status epilepticus in the ICU is heterogeneous in etiology (Figure 1). While atypical febrile seizures (febrile status epileptics) and idiopathic epilepsy were the most common presenting diagnoses, other causes included metabolic abnormalities, central nervous system infections, brain tumors, cerebral dysgenesis, AED dosing change, and drug overdose.

Figure 1 The etiology of paediatric status epilepticus at a single center PICU in Winnepeg, MB, Canada. Total number of patients (n) =189.
Risk Factor-Outcome Association
Outcome data were available on 189 patients: 132 (69.5%) returned to neurological baseline, 46 (24.3%) developed new neurological deficits, and 12 (6.3%) died in the hospital (ED, PICU) either as a consequence of SE or ensuing complications in the PICU. Risk factors associated with unfavorable outcomes of SE patients admitted to the PICU included recurrent seizures refractory to standard management, development of systemic complications of renal failure, cerebral edema, apnea during transport, and the occurrence of recurrent breakthrough seizures requiring treatment during their hospital stay (Table 1).
Table 1 Variables adversely impacting outcome in children presenting to a PICU

ICU=intensive care unit; PICU=pediatric intensive care unit
Transportation Times and Interventions in Relation to Outcome
One hundred twenty five patients were from Winnipeg (Group A; 69.44%), while 55 (30.56%) were from outside Winnipeg (Group B). Both gender distribution and age were comparable between the two groups. The time required for transport from the location of initial management to the Children’s Hospital could be determined for 121 patients (Table 2a). In addition, length of ED stay was also tabulated (Table 2a). Transportation times did not appear to influence outcome variables in the univariate analysis, despite there being a significant (expected) difference in mean transport times between patients arriving to the tertiary care center from Winnipeg versus those residents outside Winnipeg (0.45 hr vs. 2.05 hr, p<0.0001). Complications during transport that adversely affected the outcome included the occurrence of apnea (Table 2b), septic shock, and dysrhythmias. Most patients who developed new deficits experienced apnea during transport (34%). During transport, the initial AED dose was inadequate in 34 patients (Table 2b; 15.38% in Group A, 13.64% in Group B). However, most of these patients returned to baseline and did not have a significant effect on outcome. Overall, SE mortality in transport was due to respiratory distress during apneic episodes in transport or in the ED or complications from refractory SE, yet this data was not statistically significant (Table 2b).
Table 2a Transport and ED Management Duration of Patients with Status Epilepticus

Table 2b Transport and ED Complications of Patients with Seizures
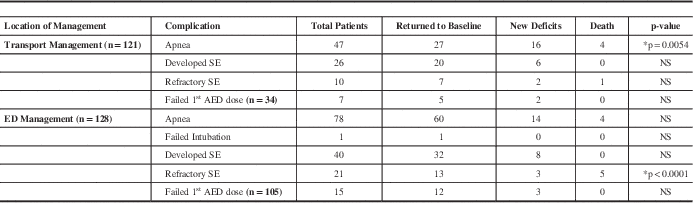
*Significant p values. NS=not significant; ED=emergency department; SE=status epilepticus; AED=anti-epileptic drugs.
Emergency Department Interventions
Patients transported from the city presented in sustained SE to the ED at a higher percentage than those transported from outside (40.91% of Group A patients vs. 9.38% Group B patients; p=0.0008). Emergency department complications are shown in Table 2b. Approximately 61% of patients experienced apnea, followed by 31% that developed SE in the ED, although they did not lead to significant outcomes. Sixteen percent of patients developed refractory SE in the ED, however, 62% returned to baseline (Table 2b). Eighteen percent of patients who developed new deficits experienced apnea in the ED, almost half the number of patients that experienced apnea during transport. Mortality of SE patients in the ED was significantly attributable to refractory SE (55%).
PICU Stay and Complications
The median duration of PICU stay was 34.38 hours (interquartile range, 16.25-59.17). The duration of transport (related to geographical distribution) did not adversely influence outcome variables. A similar proportion of patients from Winnipeg and outside of Winnipeg were admitted to the PICU on each of the three shifts (7 a.m. to 3 p.m., 3 p.m. to 11 p.m., and 11 p.m. to 7 a.m.). The time of day at which the patients were admitted was not significantly correlated with outcome. The most common indication for PICU admission was the need for mechanical ventilator support. Patient mortality, followed by new deficit development, was significantly correlated to longer duration of mechanical ventilation and therapeutic coma and increased length of hospital stay (Table 3a). Group B patients had significantly longer hospital stays (p=0.0396) and were mechanically ventilated longer (p=0.0037) than Group A counterparts residing within the city of Winnipeg (data not shown). Yet, the need for central venous access or continuous arterial pressure monitoring also correlated with poor outcomes (Table 3b). Patients with recurrent breakthrough seizures after hospitalization and those with refractory recurrent breakthrough seizures had poorer outcomes than those without recurrent seizures (Table 3b).
Table 3a Duration of PICU SE Management and Relationship to Deficit Development and Mortality

Table 3b SE Management and Complication Development in Relationship to SE Outcomes

*significant value; NS=not significant; SE=status epilepticus; AED=anti-epileptic drugs
Many of the complications in the PICU had significant effects on outcome, including renal failure, acidosis, cardiac arrhythmias, hypotension, pulmonary edema, hypoglycemia, cerebral edema, hepatic failure, disseminated intravascular coagulation, anemia, SIADH, hypertension, respiratory failure, and other complications (Table 4). The occurrence of cerebral edema and the presence of anemia were significantly associated with a poor outcome in Group B patients (p=0.0104 and p=0.0381, respectively).
Table 4 Impact of PICU Complications on SE Outcomes

NS=not significant; PICU=pediatric intensive care unit; SE=status epilepticus; DIC= Disseminated Intravascular Coagulation; ICP=intracranial pressure; AED=anti-epileptic drugs; SIADH=syndrome of inappropriate anti-diuretic hormone ; LOS=length of stay.
*Significant p values.
† n represents number of patients with specified complication out of total n=189.
‡ Total n=136. For all other complications, total n= 189.
∙ Median PICU Length of Stay (LOS) in hours with Interquartile Ranges in parentheses.
Discussion
To our knowledge, this is the first study to review outcome variables of children presenting in SE at a single-center PICU while determining its risk factors, management and overall outcome. Based on the information analyzed in the small number of patients in this cohort, we were unable to correlate etiology to PICU outcomes. Although SE has a heterogeneous etiology, children with atypical febrile seizures constitute a significantly large proportion of patients requiring intensive care. In fact, idiopathic epilepsy and febrile status epilepticus combined accounted for 50% of the patients admitted to the PICU. The incidence of SE decreases by 25-40% based on exclusion of febrile SE, indicative of its common etiology.Reference Singh and Gaillard 17 In our study, etiologies could be surmised from the evolution of illness in our PICU patients. Infection was a major complication for 87 of 189 patients (Table 3). Although there was no significant effect on outcome and most patients returned to baseline, this data does offer insight into common etiologies of SE. In a prospective cohort of patients with febrile SE reported by Shinnar et al.,Reference Shinnar, Pellock and Berg 18 the short-term outcome was considered good because the morbidity and mortality were low. It is known that viral triggers are associated with febrile SE, however, recent reports link infection with afebrile SE.Reference Juntunen, Herrgård and Mannonen 19 In our cohort of patients admitted to the PICU, 9% were identified as having a primary central nervous system infection (Figure 1). Although infectious agents were not identified, our data is consistent with studies demonstrating that several viruses, including human herpes virus-6, human herpes virus-7 and others, are causes of febrile SE.Reference Juntunen, Herrgård and Mannonen 19 While the immediate outcomes of SE following such primary infections appear to be good, the long-term outcomes with regard to behavior, cognitive function, and the delayed development of epilepsy remain unclear. Paediatric post-traumatic brain injury patients are at increased risk of subclinical and clinical SE detected via continuous EEG monitoring.Reference Arndt, Lerner and Matsumoto 20 Brain injury sequelae include PICU complications seen in this study – seizures evolving to SE, increased inracranial pressure, hyperthermia, cerebral edema, and SIADH,Reference Agha, Phillips and O’Kelly 21 - Reference Walker, Harting and Baumgartner 23 which significantly attributed to SE outcomes. It is unknown at this time if patients in our study manifested these complications based upon an established diagnosis of traumatic or anoxic brain injury, although 4% of our cases occurred after trauma (Figure 1). However, in retrospect, it is suspected that they developed aforementioned sequelae, including SE, due to brain injury.
Aggressive first-line treatment to stabilize patients in SE has improved yet still remains challenging, given multiple etiologies, e.g. 27% of our patients presented in febrile SE (Figure 1), possibly related to prolonged time in transport or lack of immediate administration of AED. The addition of extraneous variables, including prolonged transport times and failure of AED administration tend to complicate treatment in the ED and can lead to subsequent PICU admission. Transport times, in our study, was not significant in determining outcomes, likely due to the number of patient records reviewed. Despite the p-values, it is yet noteworthy to mention that longer transport times may still lead to development of new deficits in patients. Additionally, transport apnea was a significant complication (Table 1). Apnea is a known complication of emergency transport and can lead to increased mortality.Reference Nakayama, Gardner and Rowe 24 Future retrospective studies with a larger cohort might yield more insight into the etiology, as well as transport times and time to first AED administration as possible risk factors for SE onset and development of SE and complications.
During ED and PICU management of SE, refractory and recurrent SE were significant complications throughout the hospital course that adversely affect outcomes (Tables 1b and 2b). The mortality rate of patients who develop in-hospital SE vary from 32%-60%, however, there are no studies comparing this to mortality rates of those who develop out-of-hospital SE.Reference Sutter, Kaplan and Ruegg 25 There is virtually no data supporting that the incidence of prolonged and refractory SE may be influenced by geographic location (country- or region-specific data) and that the cause of SE cannot be ascertained based on increased transport times to a medical facility from a rural area.
Despite differences in initial treatments provided at the patient’s geographic location, rates of admission to the PICU did not differ. Roughly 15% of all patients received inadequate dosages of their AED medications, at the site of initial treatment as well as in the ED. In addition to inadequate dosing, a significant proportion of children admitted to the PICU received not only inadequate doses of the rescue medication (benzodiazepine) but subsequent doses of first and second line AEDs administered during transport and in the ED. The reasons for this remain unclear, but could be related to caution regarding the problem of respiratory depression secondary to the use of combinations of benzodiazepines and phenobarbital. As this phenomenon was not consistently observed, variables such as individual physician preferences, calculations, and weight errors may exist in treatment administration. All treatment protocols issued to parents and used by the prescribing practice, by transport teams, in the ED, and the PICU should emphasize the importance of ascertaining patient weight accurately in order to reduce the chances of inadequate treatment. Inadequate dosing may have been an unrecognized variable that influenced the occurrence of self-sustained SE. This observation has been made in other studies.Reference Chin, Neville and Peckham 11
The development of refractoriness to AED therapy remains one of the enduring challenges in the management of SE, especially in the ICU setting.Reference Holtkamp, Othman and Buchheim 26 , Reference Drislane, Lopez and Blum 27 In our patient cohort, we identified 17 (9%) patients admitted to the PICU who evolved to a state of treatment-refractory status epilepticus. Of these, eight (~50%) required the induction of therapeutic coma using pentobarbital. Four patients were left with fresh neurological deficits, and two patients died. We used high-dose suppression therapy such as pentobarbital and midazolam with EEG monitoring for management of refractory SE as suggested for treatment refractoriness to conventional measures.Reference Wilkes and Tasker 15 , Reference Wilkes and Tasker 28 The role of newer therapeutic alternatives for the management of status epilepticus has been described.Reference Wilkes and Tasker 15 , Reference Wheless 29 Several case series and open label trials have reported success with use of AEDs including intravenous valproic acid,Reference Wheless and Treiman 30 - Reference Yu, Mills and Thompson 32 topiramateReference Perry, Holt and Sladky 33 - Reference Kahriman, Minecan and Kutluay 35 and levetiracetam.Reference Wheless and Treiman 30 , Reference Wheless, Clarke and Carpenter 36 , Reference Patel, Landan and Levin 37 Increasing experience with these may provide additional options, prior to the consideration of high-dose suppression therapy in the ICU. Other paediatric SE studies suggest that the outcome of patients treated with high-dose suppression therapy is independent of the specific coma-inducing agents or the extent of EEG burst suppression, suggesting that the primary etiology may be a key determinant of outcome.Reference Rossetti, Logroscino and Bromfield 38 Additionally, it is known that pentobarbital causes hypotension and is associated with nosocomial infections due to white blood cell dysfunction.Reference Pugin, Foreman and De Marchis 39
Paediatric ICU complications associated with adverse outcomes include infection, cerebral edema, increased ICP, and renal failure. Infection has already been discussed as a complication of the etiology of the seizure. Increased ICP, SIADH, and cerebral edema are all related to SE etiology, specifically brain injury. Renal failure is a less specific seizure complication, but significant nevertheless to SE outcomes in this study. Acute renal failure is a commonly associated complication of seizures and SE, likely due to rhabdomyolysis from increased muscular activity during seizure. Moreover, infection increases susceptibility to acute renal failure.Reference Huerta-Alardin, Varon and Marik 40 Creatine kinase levels were not measured in any of the patients in our study, especially with either increased refractory or recurrent seizures, however, it is likely that these patients developed renal failure. The cause of renal failure in our patients is unknown but likely secondary to the seizure and musculoskeletal overactivity. With increased confidence intervals for renal failure and cerebral edema odds ratios, more data would need to be collected in order to determine if renal failure and cerebral edema are variables truly indicative of worsening outcomes.
There are few studies of long-term outcomes among children who experienced SE requiring PICU admission, although it has been reported that children with EEG-monitored status epilepticus have worsened outcomes.Reference Tasker 41 In general, our retrospective data point to a favorable outcome for the majority (~70%), and considerable morbidity in the form of added neurological deficits (24%), with mortality rates that are very similar to other reported studies.Reference Chin, Neville and Peckham 11 The risk of subsequent epilepsy (13-74%), and of recurrent SE (3-56%) varies across published studies and meta-analyses.Reference Chin, Neville and Scott 42
There are several limitations to this study: inclusion of a small number of subjects, limiting the statistical analysis; incomplete or missing datasets; a selection bias towards the sickest patients in SE; seizure types that could not be classified accurately from the abstracted data; unclassified types of SE; outcomes that were chosen upon discharge leading to deficits that may not have been recorded; failure to correlate longer transport times and PICU stay with new deficits acquired during transport; and, finally, long-term follow-up outcomes with deficit specification remain unreported. Additionally, in Table 2a, new deficits acquired during transport did not correlate with longer transport times and PICU stays. However, this would be better addressed with a prospective analysis.
Conclusions
Our clinical findings impart a sense of urgent need to establish risk factors of paediatric SE and its complications during the hospital course, recognize the challenges in the management of paediatric status epilepticus to prevent the significant morbidity associated with epilepsy, as well as the long-term comorbidities in the form of behavioral and neurocognitive impairments.
Acknowledgments
The authors thank the B. Sc. Medicine program at the University of Manitoba for the stipendiary support for Dr. R. Johnson; the Manitoba Institute of Child Health (Monagle, #33) and the Children’s Hospital Foundation of Manitoba for a their support and Ms. Pamela Cate and Mrs. Andrea Patters for their editorial assistance.
Disclosures
Samir Shah, Namrata Shah, Robert Johnson, Alina Nico West, and Narayan Prasad do not have anything to disclose.
No financial support was received for this study.











