Article contents
Calculated melt and restite compositions of some Australian granites
Published online by Cambridge University Press: 03 November 2011
Abstract
The thermodynamic relations embodied in the Quasicrystalline Model of Burnham and Nekvasil (1986), as recently extended by the author, have been used to quantitatively assess the feldspar-quartz liquidus relations in two I-type (Jindabyne and Moruya) and two S-type (Bullenbalong and Dalgety) suites of Australian granites, using analytical data provided by B. W. Chappell and co-workers. Among the more notable results obtained from these calculations at a constant pressure of 5·0kbar and 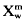 = 0·30 (≍2·8 wt% H2O), for purposes of comparison, are that: (1) felsic melts of remarkably uniform, but distinctive composition can be extracted from each suite, leaving solid residues in amounts up to 65 mol%; (2) all melts from both S-type suites have two feldspars plus quartz on their liquidii, whereas both I-type suites have only plagioclase plus quartz on their liquidii; (3) the total solid residue ranges from 27-63% in the Jindabyne suite, from 15–62% in the Moruya suite, from 30–65% in the Bullenbalong suite, and from 27–65% in the Dalgety suite; (4) liquidus temperatures of the S-type Bullenbalong and Dalgety melts are similar (856° and 860°C), reflecting similar feldspar compositions of An53, Or75 and An60, Or77, respectively; (5) liquidus temperatures of the I-type Jindabyne and Moruya melts, however, are distinctly different (950° and 894°C), reflecting correspondingly different plagioclase compositions of An80 and An52; (6) the calculated liquidus plagioclase composition throughout a given suite is very uniform (±1%) and amounts to as much as 46% of the total rock; and (7) these calculated liquidus and residual plagioclase compositions are also the same, within the uncertainty of measurement, as those of the plagioclase crystal-cores determined optically by A. J. R. White. The only plausible explanation for this remarkable consanguinity in plagioclase liquidus, residue, and crystal-core compositions, hence liquidus temperatures, is that the bulk of the residue is restite, in accordance with the model of White and Chappell (1977). This explanation is corroborated by the very systematic variations in the amounts of individual restite minerals with respect to total restite contents. Accordingly, those members of each suite that contain more than 60% total restite probably closely represent the bulk composition of the source rock, which is dioritic or andesitic for the Jindabyne suite, tonalitic or dacitic for the Moruya suite, pelitic metagreywacke for the Bullenbalong suite, and feldspathic metagreywacke for the Dalgety suite. As a corollary, those members with less than 60% restite must have undergone melt-restite segregation (unmixing), probably during ascent and emplacement.
= 0·30 (≍2·8 wt% H2O), for purposes of comparison, are that: (1) felsic melts of remarkably uniform, but distinctive composition can be extracted from each suite, leaving solid residues in amounts up to 65 mol%; (2) all melts from both S-type suites have two feldspars plus quartz on their liquidii, whereas both I-type suites have only plagioclase plus quartz on their liquidii; (3) the total solid residue ranges from 27-63% in the Jindabyne suite, from 15–62% in the Moruya suite, from 30–65% in the Bullenbalong suite, and from 27–65% in the Dalgety suite; (4) liquidus temperatures of the S-type Bullenbalong and Dalgety melts are similar (856° and 860°C), reflecting similar feldspar compositions of An53, Or75 and An60, Or77, respectively; (5) liquidus temperatures of the I-type Jindabyne and Moruya melts, however, are distinctly different (950° and 894°C), reflecting correspondingly different plagioclase compositions of An80 and An52; (6) the calculated liquidus plagioclase composition throughout a given suite is very uniform (±1%) and amounts to as much as 46% of the total rock; and (7) these calculated liquidus and residual plagioclase compositions are also the same, within the uncertainty of measurement, as those of the plagioclase crystal-cores determined optically by A. J. R. White. The only plausible explanation for this remarkable consanguinity in plagioclase liquidus, residue, and crystal-core compositions, hence liquidus temperatures, is that the bulk of the residue is restite, in accordance with the model of White and Chappell (1977). This explanation is corroborated by the very systematic variations in the amounts of individual restite minerals with respect to total restite contents. Accordingly, those members of each suite that contain more than 60% total restite probably closely represent the bulk composition of the source rock, which is dioritic or andesitic for the Jindabyne suite, tonalitic or dacitic for the Moruya suite, pelitic metagreywacke for the Bullenbalong suite, and feldspathic metagreywacke for the Dalgety suite. As a corollary, those members with less than 60% restite must have undergone melt-restite segregation (unmixing), probably during ascent and emplacement.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © Royal Society of Edinburgh 1992
References
- 7
- Cited by




