Article contents
High diversity of trematode metacercariae that parasitize freshwater gastropods in Bangkok, Thailand, and their infective situations, morphologies and phylogenetic relationships
Published online by Cambridge University Press: 10 March 2022
Abstract
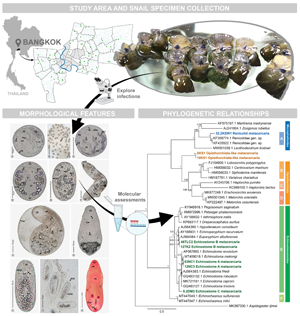
We investigated diversity, infective situations, morphological features and phylogenetic relationships of the metacercariae in freshwater snails from Bangkok between March 2018 and February 2020. Crushing and dissection techniques were performed to explore the metacercariae in the snail hosts. Polymerase chain reaction was implemented to amplify the internal transcribed spacer 1 (ITS1), 5.8S ribosomal DNA and ITS2 regions of metacercarial DNA. A total of 3173 of all 21 707 snails showed infections with metacercariae, representing a relatively high infective prevalence (14.62%) compared to earlier research. All infected snails belonged to 14 species/subspecies. A group of viviparid snails exhibited the highest metacercarial infections (26.10–82.18%). We found metacercariae with seven morphological groups. Five of them can be stated as new records of the metacercariae in Thailand, indicating a broader spectrum of larval trematode diversity. Our phylogenetic assessments established that five of the seven morphological groups can be molecularly classified into different taxonomic levels of digenean trematodes. Echinostome A metacercariae revealed the highest infective prevalence (7.15%), and their sequence data were conspecific with a sequence of Echinostoma mekongki, which is a human intestinal fluke; this finding denotes the distribution and suggests epidemiological surveillance of this medically important fluke in Bangkok and adjacent areas. However, two groups of Opisthorchiata-like and renicolid metacercariae remain unclear as to their narrow taxonomic status, although their molecular properties were considered. For more understanding about trematode transmissions in ecosystems, both physical and biological factors may be further analysed to consider the factors that relate to and contribute to trematode infections.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press
References
- 1
- Cited by





