Obesity is characterised by an increase in white adipose tissue (WAT) mass, which can result from an excess of food intake relative to energy expenditure. Obesity is also associated with hepatic steatosis and hyperlipidaemia, indicated by excessive accumulation of fat in the liver and excess fat in circulation( Reference Haslam and James 1 ). Central adiposity due to accumulation of visceral adipose tissue is linked to significantly increased risk of hepatic steatosis( Reference Thomas, Hamilton and Patel 2 ). Increased NEFA flux from visceral fat to the liver is one of the suggested underlying mechanisms( Reference Kuriyama, Yamashita and Shimomura 3 ). Most dietary approaches for obesity prevention or treatment attempt to limit consumption of a diet rich in saturated fat( Reference Walker, Zariwala and Holness 4 ). In contrast, an increase in fruit and vegetable intake can help decrease obesity, which may be attributable to their low-energy density, fibre content or phenolic compound enrichment( Reference Astrup, Dyerberg and Selleck 5 , Reference Rastmanesh 6 ).
Resveratrol (RV, 3,5,4′-trihydroxystilbene) is one of the natural polyphenolic compounds mainly found in grape skin. RV is well known for its cardioprotective( Reference Hung, Chen and Huang 7 ), anti-cancer( Reference El-Mowafy and Alkhalaf 8 ), anti-inflammatory, antioxidant( Reference de la Lastra and Villegas 9 ) and phyto-oestrogenic( Reference Bowers, Tyulmenkov and Jernigan 10 ) properties. Some studies have suggested that RV may also have beneficial effects on obesity. For example, RV appears to inhibit the proliferation of pre-adipocytes in vitro ( Reference Hsu and Yen 11 ), and hence may suppress the production of new fat cells. In addition, RV appears to inhibit lipogenesis as well as lipid accumulation( Reference Shang, Chen and Xiao 12 ) in human liver cells and mature adipocytes( Reference Fischer-Posovszky, Kukulus and Tews 13 , Reference Szkudelska, Nogowski and Szkudelski 14 ). While the evidence from cellular studies suggests that RV may suppress the production of new fat cells and prevent fat accumulation in liver cells and adipocytes, the effects of RV on obesity and steatosis in vivo have been far more equivocal( Reference Lagouge, Argmann and Grhart-Hines 15 – Reference Baur, Pearson and Price 20 ).
Several in vivo studies reported that RV protected against diet-induced obesity( Reference Lagouge, Argmann and Grhart-Hines 15 , Reference Kim, Jin and Choi 16 ) and hepatic steatosis( Reference Shang, Chen and Xiao 17 ) in murine models. For example, body weight gain and fat accumulation were suppressed in mice fed a high-fat diet (HFD) with high dietary RV intake (400 mg/kg body weight per d) over 15 weeks. In these HFD-fed mice, RV up-regulated the silent information regulating 2 homologue 1 (SIRT1) gene, which is a key regulator of energy and metabolic homeostasis( Reference Lagouge, Argmann and Grhart-Hines 15 ). However, in other studies, RV was reported to have minimal or no effect on obesity and hepatic steatosis( Reference Rivera, Morón and Zarzuelo 18 – Reference Baur, Pearson and Price 20 ). In obese Zucker rats, RV supplementation (10 mg/kg body weight per d) over 8 weeks failed to reduce body weight, although RV reduced adipose tissue mass( Reference Rivera, Morón and Zarzuelo 18 ). Similarly in mice, RV (22·4 mg/kg body weight per d) supplementation for 15 months had no effect on body weight, although RV appeared to mimic the effects of energy restriction( Reference Baur, Pearson and Price 20 ). The lack of consensus on the in vivo effect of RV on diet-induced obesity and hepatic steatosis may have been due to varying doses, diet and duration in previous studies. Importantly, the potential mechanism underlying the anti-obesity and hepatoprotective effects of RV in vivo still remains unclear. In particular, few studies have determined the activities of enzymes involved in lipogenesis and fatty acid (FA) oxidation in the liver and adipose tissue of RV-supplemented, diet-induced obese mice.
Accordingly, we first determined whether RV (0·005 or 0·02 % in diet) had any dose-dependent effects on body weight gain, visceral adiposity, hepatic steatosis and dyslipidaemia in HFD-fed mice, a well-established murine model of diet-induced obesity( Reference West, Boozer and Moody 21 , Reference Ghibaudi, Cook and Farley 22 ). Secondly, we examined lipid metabolic enzyme activity in the liver and WAT to further elucidate potential underlying mechanisms responsible for the effects of RV on diet-induced obesity.
Experimental methods
Animals and diets
A total of forty male C57BL/6J mice (4 weeks old) were purchased from the Jackson Laboratory. The animals were maintained in a room with controlled temperature (20–23°C) and lighting (alternating 12 h periods of light and dark) and fed a pelletised commercial non-purified diet for 1 week after arrival. The mice were then randomly divided into four groups (n 10) and fed the respective experimental diets for 10 weeks, as shown in Table 1; normal diet (ND) control (ND, American Institute of Nutrition (AIN)-76 semisynthetic diet), HFD control (HFD, 20 % fat based on the AIN-76 diet plus 1 % cholesterol, w/w), 0·02 % RV (HFD+high RV, 0·02 % RV with HFD, w/w, Sigma Chemical Company) and 0·005 % RV (HFD+low RV, w/w, 0·005 % RV with HFD, w/w). The HFD was formulated to provide 20 % of the total energy from fat, by replacing carbohydrate energy with lard and maize oil, and had the same amount of vitamins and minerals per kJ as the ND. The mice had free access to food and distilled water during the experimental period. Food intake and body weight were measured daily. All animal procedures were approved by the Ethics Committee for animal studies at Kyungpook National University, Republic of Korea.
Table 1 Composition of experimental diets (% of diet, w/w)
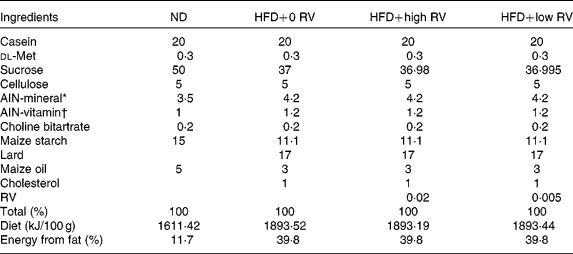
ND, normal diet; HFD, high-fat diet; RV, resveratrol; HFD+0 RV, HFD; HFD+high RV, HFD supplemented with 0·02 % RV; HFD+low RV, HFD supplemented with 0·005 % RV; AIN, American Institute of Nutrition.
* AIN-76 mineral mixture: calcium phosphate 500 g/kg, NaCl 74 g/kg, potassium citrate 2220 g/kg, potassium sulphate 52 g/kg, magnesium oxide 24 g/kg, manganous carbonate 3·5 g/kg, ferric citrate 6 g/kg, zinc carbonate 1·6 g/kg, cupric carbonate 0·3 g/kg, potassium iodate 0·01 g/kg, sodium selenite 0·01 g/kg, chromium potassium sulphate 0·55 g/kg, sucrose 118·03 g/kg.
† AIN-76 vitamin mixture: thiamin HCl 0·6 g/kg, riboflavin 0·6 g/kg, pyridoxine HCl 0·7 g/kg, niacin 3 g/kg, calcium pantothenate 1·6 g/kg, folic acid 0·2 g/kg, biotin 0·02 g/kg, vitamin B12 1 g/kg, vitamin A (500 000 IU/g) 0·8 g/kg, vitamin D3 (400 000 IU/g) 0·25 g/kg, vitamin E acetate (500 IU/g) 10 g/kg, menadione sodium bisulphite 0·08 g/kg, sucrose 981·15 g/kg.
Sampling
At the end of the experimental period, the mice were anaesthetised with ketamine after a 12 h fast. Blood samples were collected in heparinised tubes from the inferior vena cava and stored at − 70°C before analysis of plasma biomarkers. The liver and visceral fat depots (epididymal, perirenal, mesentery and retroperitoneum) were removed, rinsed with a physiological saline solution, weighed and immediately stored at − 70°C.
Lipid analyses
Plasma NEFA were measured using a commercial assay kit (NEFA-Wako; Wako Pure Chemical Industries). Plasma TAG and total cholesterol were measured using commercial kits (Asan Pharmaceutical), based on the lipase–glycerol phosphate oxidase method( Reference McGowan, Artiss and Strandbergh 23 ) and cholesterol oxidase method, respectively( Reference Allain, Poon and Chan 24 ). Plasma apoB was also determined using a commercial kit (Nitto Boseki).
Hepatic lipids were extracted using the method of Folch et al. ( Reference Folch, Lees and Sloane Stanley 25 ). The dried lipid residues were dissolved in 1 ml of ethanol for cholesterol and TAG assays. Triton X-100 and sodium cholate solution were added to 200 μl of the dissolved lipid solution to produce a final concentration of 3 mm. The hepatic cholesterol and TAG levels were analysed with the same commercial kit as used in the plasma analysis (Asan Pharmaceutical).
Lipid-regulating enzyme activities
To measure lipid-regulating enzyme activities in the liver and epididymal WAT, enzyme sources were prepared according to the method reported by Hulcher & Oleson( Reference Hulcher and Oleson 26 ). FA synthase (FAS) activity was determined by a spectrophotometric assay according to the methods of Carl et al. ( Reference Carl, Lakshmanan and Porter 27 ); one unit of FAS activity represented the oxidation of 1 nmol of NADPH per min at 30°C. The glucose-6-phosphate dehydrogenase (G6PD) activity was determined using the method of Pitkänen et al. ( Reference Pitkänen, Pitkänen and Uotila 28 ), based on the reduction of 1 nmol NADP per min at 25°C measured at 340 nm using a spectrophotometer. Phosphatidate phosphohydrolase (PAP) activity was measured using the method of Walton & Possmayer( Reference Walton and Possmayer 29 ) and PAP activity was expressed as nmol/min per mg protein. FA β-oxidation was measured spectrophotometrically by monitoring the reduction of NAD to NADH in the presence of palmitoyl-CoA as described by Lazarow( Reference Lazarow 30 ). The amount of protein in enzyme sources was determined by the Bradford method( Reference Bradford 31 ) using bovine serum albumin as the standard.
Histopathological analysis
Liver and epididymal WAT were removed from the mice and fixed in a buffer solution of 10 % formalin. All fixed tissues were processed routinely for paraffin embedding and 4 μm sections were prepared and stained with haematoxylin and eosin. Stained areas were viewed using an optical microscope (Zeiss Axioscope) with a magnifying power of × 200 and epididymal adipocyte size was measured by using Motic Images Plus 2.0ML (Motic).
Statistical analysis
All data were presented as the mean and standard error. Statistical analysis was performed using software SPSS (version 11.0, SPSS, Inc.). Statistical differences between ND and HFD results were determined using Student's t test. One-way ANOVA was performed to compare HFD groups with or without RV and Tukey's post hoc test was performed when significant differences were identified between the groups at P < 0·05.
Results
Effects of resveratrol on body weight and food intake in diet-induced obese mice
Food intake was significantly suppressed in mice fed a HFD alone compared to ND-fed mice, and daily energy intake was not different between the two groups (Fig. 1). However, body weight was significantly greater in the mice fed a HFD alone compared to ND-fed mice after 6 weeks. In HFD-fed mice, supplementation of RV at a low dose (0·005 %) significantly suppressed body weight gain after 3 weeks, but the higher dose of RV (0·02 %) was surprisingly not effective (Fig. 1). Neither food intake nor energy intake was affected by RV supplementation, whereas food efficiency ratio was significantly lower in the low-dose (0·005 %) RV group, compared to the HFD control group (Fig. 1).

Fig. 1 Effects of resveratrol (RV) supplementation on (A) body weight (
![]() , normal diet (ND);
, normal diet (ND);
![]() , high-fat diet (HFD, HFD+0 RV);
, high-fat diet (HFD, HFD+0 RV);
![]() , HFD supplemented with 0·02 % RV (HFD+high RV);
, HFD supplemented with 0·02 % RV (HFD+high RV);
![]() , HFD supplemented with 0·005 % RV (HFD+low RV)), (B) visceral white adipose tissue (WAT) (□, ND; ■, HFD+0 RV;
, HFD supplemented with 0·005 % RV (HFD+low RV)), (B) visceral white adipose tissue (WAT) (□, ND; ■, HFD+0 RV;
![]() , HFD+high RV;
, HFD+high RV;
![]() , HFD+low RV), (C) food intake weight, (D) food efficiency ratio (FER) and (E) energy intake in C57BL/6J mice fed a HFD. Values are means, with their standard errors represented by vertical bars. a,b,c Mean values with unlike letters are significantly different among groups (P < 0·05). Mean values were significantly different for ND from those of HFD: * P < 0·05, ** P < 0·01, *** P < 0·001. Food efficiency ratio (FER) = body weight gain/food intake.
, HFD+low RV), (C) food intake weight, (D) food efficiency ratio (FER) and (E) energy intake in C57BL/6J mice fed a HFD. Values are means, with their standard errors represented by vertical bars. a,b,c Mean values with unlike letters are significantly different among groups (P < 0·05). Mean values were significantly different for ND from those of HFD: * P < 0·05, ** P < 0·01, *** P < 0·001. Food efficiency ratio (FER) = body weight gain/food intake.
Effects of resveratrol on fat accumulation in diet-induced obese mice
HFD feeding resulted in a significant increase in total visceral WAT weight, including the epididymal, perirenal, retroperitoneal and mesentery depots compared to ND (Fig. 1). RV intake had differential effects on visceral adipose depots. Perirenal and mesentery adipose depots were significantly reduced in HFD-fed mice supplemented with either high-dose or low-dose RV (Fig. 1). Only the low dose of RV (0·005 %) effectively reduced the weight of the epididymal and retroperitoneal adipose depots in HFD-fed mice (Fig. 1). Hence, overall total visceral adipose weight was significantly lowered by RV supplementation, although the low dose appeared to be more effective than the higher dose (Fig. 1). Morphological observations also indicated that epididymal adipocyte size was smaller in the RV-supplemented mice than in the HFD control mice (Fig. 2(B) and (C)).

Fig. 2 Effects of resveratrol (RV) supplementation on (A) liver and (B) epididymal adipose tissue morphology in C57BL/6J mice fed a high-fat diet (HFD). Haematoxylin and eosin (H&E)-stained transverse-section of the liver and epididymal fat; each liver and epididymal fat (n 10) were removed and wrapped with saline-soaked gauze after removing the connective tissues. All were fixed in 10 % parafomaldehyde/PBS, embedded in paraffin, and then stained with H&E. Original magnification × 200. Effects of RV supplementation on epididymal adipocyte size in C57BL/6J mice fed a HFD. Values are means, with their standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05). *** Mean value was significantly different from those of the HFD groups (P < 0·001). ND, normal diet; HFD+0 RV, HFD; HFD+high RV, HFD supplemented with 0·02 % RV; HFD+low RV, HFD supplemented with 0·005 % RV. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Effect of resveratrol on plasma and hepatic lipid levels in diet-induced obese mice
Next, we determined if there was any evidence of a dose-dependent effect of RV on plasma and hepatic lipid levels (Table 2). Plasma NEFA and TAG levels were not elevated in the mice fed the HFD alone compared to ND-fed mice. As a result of a concomitant increase in both plasma total cholesterol and HDL-cholesterol levels, atherogenic index was unchanged in the HFD control group compared to the ND group. However, the apoB:apoA-I ratio, which is a more sensitive biomarker to measure CHD than atherogenic index, was significantly higher in the HFD control group compared to the ND group. Despite the lack of changes in plasma lipid levels induced by the HFD, RV at both a low and high dose (0·005 and 0·02 %) significantly reduced plasma NEFA level compared to the HFD control group and the ND group. Remarkably, RV was more effective at a low dose (0·005 %), but not at a higher dose (0·02 %), for reducing plasma TAG and total cholesterol levels in HFD-fed mice. Plasma HDL-cholesterol level was not significantly different between mice fed the HFD alone and the two RV groups. However, the apoB:apoA-I ratio was significantly lower in the low-dose RV group compared to the HFD control group.
Table 2 Effects of resveratrol (RV) supplementation on plasma and hepatic lipid levels in C57BL/6J mice fed a high-fat diet (HFD) (Mean values with their standard errors)

ND, normal diet; HFD+0 RV, HFD; HFD+high RV, HFD supplemented with 0·02 % RV; HFD+low RV, HFD supplemented with 0·005 % RV; AI, atherogenic index.
a,b,c Mean values with unlike superscript letters are significantly different (P < 0·05).
Mean values were significantly different for ND from those of HFD: * P < 0·05, *** P < 0·001.
† AI = (total cholesterol − HDL-cholesterol)/HDL-cholesterol.
Hepatic TAG and cholesterol contents were increased approximately 2-fold and 6-fold, respectively, in C57BL/6 mice fed the HFD alone as previously observed by others( Reference Wierzbicki, Chabowski and Zendzian-Piotrowska 32 , Reference Oosterveer, van Dijk and Tietge 33 ). In HFD-fed mice, RV significantly reduced hepatic TAG and cholesterol contents compared to the HFD control group, although not in a dose-dependent manner. Consistent with the observed hepatic TAG and cholesterol contents, haematoxylin and eosin staining of liver sections for lipids indicated that hepatic lipid accumulation was more pronounced in the HFD control mice compared to ND-fed mice (Fig. 2). RV caused a marked decrease in the number and size of liver fat droplets, and the low dose of RV (0·005 %) appeared to be more effective than the higher dose of RV (0·02 %).
Effect of resveratrol on lipid-regulating enzyme activities in liver of diet-induced obese mice
To further examine the mechanism via which RV suppresses lipid accumulation in the liver, we determined the activity of hepatic lipid-regulating enzymes. The activity of hepatic PAP, which is a rate-limiting enzyme in TAG synthesis, was elevated in mice fed the HFD alone compared to ND-fed mice. We observed no changes in de novo lipogenic FAS and G6PD activities in the liver of mice fed the HFD alone. However, FA oxidation was significantly augmented in the mice fed the HFD alone compared to ND-fed mice (Table 3). In contrast, the activity of PAP was significantly suppressed by RV in the liver of HFD-fed mice without affecting FA oxidation (Table 3). Furthermore, 0·02 % RV in the diet significantly decreased hepatic FAS activity by 43 % compared to the HFD alone (Table 3).
Table 3 Effects of resveratrol (RV) supplementation on lipid-regulating enzyme activities in the liver and adipose tissue of C57BL/6J mice fed a high-fat diet (HFD) (Mean values with their standard errors)

ND, normal diet; HFD+0 RV, HFD; HFD+high RV, HFD supplemented with 0·02 % RV; HFD+low RV, HFD supplemented with 0·005 % RV; FAS, fatty acid synthase; G6PD, glucose-6-phosphate dehydrogenase; PAP, phosphatidate phosphohydrolase.
a,b,c Mean values with unlike superscript letters are significantly different (P < 0·05).
Mean values were significantly different for ND from those of HFD: * P < 0·05, ** P < 0·01.
Effect of resveratrol on activities of enzymes involved in lipogenesis and fatty acid oxidation in epididymal white adipose tissue of diet-induced obese mice
We next examined the activities of enzymes that can regulate lipid accumulation in epididymal WAT. C57BL/6J mice fed the HFD alone showed a significant increase of lipogenic enzyme PAP activity in epididymal WAT compared to ND-fed mice, although no change in β-oxidation was observed in HFD-fed mice (Table 3). In contrast, RV significantly decreased FAS and PAP activities in epididymal WAT compared to the HFD-alone group (Table 3). Furthermore, mice fed a low dose of RV (0·005 %) showed a significant decrease in G6PD activity and a significant increase in FA β-oxidation in epididymal WAT compared to the mice fed the HFD alone (Table 3).
Discussion
A chronic HFD is a major contributing factor underlying the development of obesity in humans. Currently, there is widespread interest in natural compounds that can suppress diet-induced obesity. In the present study, we first determined whether RV exerted any dose-dependent effect on weight gain, hepatic steatosis or dyslipidaemia. Next, we determined whether RV affected lipid-regulating enzyme activity in the liver or adipose tissue. We used C57BL/6J mice which are prone to the development of obesity when fed a HFD, developing similar characteristics as human obesity, compared to genetically obese animals( Reference West, Boozer and Moody 21 , Reference Ghibaudi, Cook and Farley 22 , Reference Collins, Martin and Surwit 34 ). We found that RV protected against diet-induced obesity, but observed unexpectedly no dose–response effects.
As others have shown( Reference West, Boozer and Moody 21 , Reference Ghibaudi, Cook and Farley 22 , Reference Collins, Martin and Surwit 34 ), the HFD resulted in a marked increase of body weight gain due to increased adiposity, despite significantly decreased food intake. RV significantly suppressed adiposity in HFD-fed mice, due to lower visceral WAT weight. Consistent with these results, we observed that epididymal WAT size was smaller in HFD-fed mice supplemented with RV. RV supplemented at a lower dose was more effective than the higher dose at suppressing visceral fat accumulation. Furthermore, RV at a low dose, but not a high dose, significantly suppressed body weight gain compared to HFD control mice. Past animal studies have been equivocal, with some studies reporting that RV suppresses body weight gain and fat accumulation in HFD-fed mice( Reference Lagouge, Argmann and Grhart-Hines 15 , Reference Kim, Jin and Choi 16 ) and rats( Reference Shang, Chen and Xiao 17 ), while several studies have reported that RV has no effect on body weight gain( Reference Rivera, Morón and Zarzuelo 18 – Reference Baur, Pearson and Price 20 ). The discrepancies between past studies may be attributable to differences in diet composition, duration of diet, dosage and experimental animals used. Few past studies have examined any dose–response effects of RV in HFD-fed animals. One previous study in rats fed a hyperenergetic diet containing different doses (6, 30, 60 mg/kg body weight per d) of RV for 6 weeks reported no dose–response effects on adipose weight( Reference Macarulla, Alberdi and Gómez 35 ). Importantly, our study compared two doses of RV, both below 0·02 %, equivalent to 2 mg/kg body weight per d. Our findings suggest that RV may be an effective anti-obesity agent, reducing body weight and fat accumulation in response to a HFD, and that 0·005 % RV may be an adequate amount for suppressing body weight gain and adiposity, although a response study with lower doses of RV is further needed to determine the minimum effective dose.
Next, we examined potential mechanisms underlying the body weight and adiposity-suppressing effect of RV. RV did not lead to decreased food intake, because no significant difference in food intake was observed in the HFD-fed mice regardless of RV administration. Many studies have reported that dietary fat led to augmentation of lipogenic enzyme activity( Reference Nelson, Kelley and Schmidt 36 , Reference Rodríguez, Macarulla and Chávarri 37 ), although contradictory findings have been reported( Reference Jiang, Wang and Yu 38 ). WAT is a major site of de novo FA synthesis, and the FA synthesised in WAT can be either re-esterified to TAG within WAT or oxidised via mitochondrial β-oxidation along with FA from the diet. FAS plays a central role in de novo lipogenesis by catalysing the synthesis of saturated long-chain FA from acetyl-CoA, malonyl-CoA and NADPH. G6PD is also a lipogenic enzyme involved in supplying NADPH for the biosynthesis of FA. In the present study, no changes in FAS or G6PD activity as well as FA β-oxidation activity were observed in epididymal WAT of ND-fed mice compared to mice fed a HFD alone. However, we observed a significant increase in PAP activity, a rate-limiting enzyme in TAG synthesis in epididymal WAT. These findings indicate that HFD does not promote de novo FA synthesis and fat oxidation in adipose tissue. The increased epididymal WAT mass in HFD-fed mice may be attributable to increased esterification of NEFA provided by the diet. Interestingly, in HFD-fed mice, RV administration significantly inhibited the activity of de novo lipogenic enzymes including FAS and G6PD as well as PAP in epididymal WAT. Again we observed no dose–response effect, and 0·005 % RV significantly augmented FA β-oxidation in epididymal WAT, which may help explain the lower visceral adiposity compared to 0·02 % RV-supplemented mice. Our findings are consistent with a previous study that reported suppression of FAS gene expression and protein level by RV in epididymal WAT of HFD-fed mice( Reference Kim, Jin and Choi 39 ). Furthermore, RV has been reported to inhibit de novo lipogenesis in parallel with the down-regulation of lipogenic gene expression in human adipocytes( Reference Fischer-Posovszky, Kukulus and Tews 13 ). Taken together, the current evidence indicates that the suppression of adiposity by RV may be due to the inhibition of de novo FA synthesis or stimulation of FA oxidation in WAT.
Another possible mechanism by which dietary RV ameliorates adiposity may be due to the activation of lipolysis. Lipolysis is one of the important metabolic pathways regulating TAG accumulation in WAT and two major enzymes, hormone-sensitive lipase and adipose TAG lipase, are involved in adipose TAG hydrolysis. Rayalam et al. ( Reference Rayalam, Yang and Ambati 40 ) have shown that RV down-regulated the expression of hormone-sensitive lipase as well as lipogenic gene in WAT, indicating that RV may alter fat mass by directly affecting the biochemical pathways involved in adipogenesis. In contrast, a recent study reported that RV induced the release of NEFA but not glycerol in 3T3 L1 cells and up-regulated adipose TAG lipase mRNA and protein expression without affecting hormone-sensitive lipase( Reference Lasa, Schweiger and Kotzbeck 41 ). Furthermore, NEFA release was increased by RV in epididymal WAT of wild-type and hormone-sensitive lipase knockout mice, but no changes were observed in epididymal WAT of adipose TAG lipase knockout mice, suggesting that adipose TAG lipase seems to be the main target for the lipolytic effect of RV( Reference Lasa, Schweiger and Kotzbeck 41 ).
Fat accumulation in adipose tissue is closely associated with the development of hepatic steatosis, which is an excessive accumulation of fat in the liver. Several mechanisms may lead to a hepatic steatosis: (1) increased NEFA supply due to increased lipolysis from WAT and/or increased intake of dietary fat, (2) increased de novo hepatic lipogenesis, (3) decreased fatty oxidation in the liver and (4) decreased hepatic VLDL–TAG secretion( Reference Duval, Muller and Kersten 42 ). Excessive consumption of dietary fat, especially SFA, leads to an excess accumulation of TAG in the liver( Reference Wierzbicki, Chabowski and Zendzian-Piotrowska 32 , Reference Oosterveer, van Dijk and Tietge 33 ). We also observed that the HFD led to a significant increase of hepatic TAG content as well as hepatic PAP activity, consistent with a previous observation that SFA dramatically increased PAP gene expression( Reference Martin, Guillou and Lasserre 43 ). However, the HFD did not lead to increased de novo lipogenic FAS and G6PD activities in the liver, or lead to increased plasma NEFA and TAG levels compared to ND-fed mice. In agreement with the present study, plasma NEFA and TAG levels, as well as hepatic FAS activity and FAS gene expression, were not increased in HFD (38·1 % fat, w/w) -fed C57BL/6 mice after 18 weeks( Reference Sato, Kawano and Notsu 44 ). Furthermore, Kim et al. ( Reference Kim, Sohn and Ahn 45 ) reported that a HFD did not affect plasma NEFA and TAG levels in C57BL/6 mice, although the HFD induced hepatic steatosis and adiposity. Moreover, Kim et al. ( Reference Kim, Sohn and Ahn 45 ) showed that genes related to FA β-oxidation were activated in the liver of HFD-fed mice, consistent with our data. Accordingly, these findings suggest that a HFD can promote hepatic lipogenesis by esterification of NEFA provided from the diet but not from de novo lipogenesis in the liver. Hepatic TAG accumulation may lead to augmentation of FA oxidation by a feedback mechanism. We found that RV significantly reduced hepatic steatosis and TAG content as well as plasma NEFA level in HFD-fed mice. The suppression of hepatic lipogenesis by RV was linked to decreased activities of hepatic de novo lipogenic FAS as well as PAP without affecting FA oxidation. The low dose of RV appeared to be more effective for suppressing hepatic steatosis, similar to body weight and fat. Taken together, these findings suggest that the beneficial effect of RV on hepatic steatosis is mediated, at least in part, by a decrease in de novo FA synthesis and esterification based on the evidence of lower hepatic FAS and PAP activity. Previous studies also indicate that RV may prevent hepatic steatosis by regulating hepatic lipid metabolism-related genes such as FAS and SIRT1 ( Reference Ajmo, Liang and Rogers 46 ). Also, the lowered plasma NEFA may account in part for the decrease in hepatic TAG content in mice supplemented with RV, since hepatic TAG accumulation during insulin resistance occurs as a result of the increase in the supply of circulating NEFA to the liver as well as through an increased endogenous lipogenesis( Reference Duval, Muller and Kersten 42 ).
In the present study, we observed that the HFD resulted in an increased plasma apoB:apoA-I ratio, as well as total cholesterol concentration mostly due to increased HDL-cholesterol concentration. The increase in total cholesterol and HDL-cholesterol is a common feature of most mouse strains fed a HFD( Reference Paigen 47 ). Plasma apoB or apoA-I concentrations independently or together as a ratio (apoB:apoA-I) are reported to predict cardiovascular risk more accurately than lipid levels( Reference Davidson 48 ). Previously we reported that long-term RV supplementation over 20 weeks significantly decreased plasma total cholesterol and LDL-cholesterol levels in apoE knockout mice fed an ND, whereas the apoB:apoA-I ratio was increased( Reference Do, Kwon and Kim 49 ). Herein, we observed that 0·005 % RV normalised the HFD-mediated increase of plasma total cholesterol and apoB:apoA-I ratio, whereas the beneficial effects were not observed in HFD-fed mice supplemented with 0·02 % RV. Zern et al. ( Reference Zern, West and Fernandez 50 ) reported that the grape polyphenol failed to improve plasma total cholesterol level, although hepatic cholesterol metabolism was altered based on evidence of lower hepatic acyl-CoA:cholesterol acyltransferase (ACAT) activity, a key cholesterol-regulating enzyme involved in the esterification and absorption of cholesterol( Reference Suckling and Stange 51 ). Furthermore, grape polyphenol was suggested to decrease the secretion of hepatic LDL-cholesterol reducing cholesterol accumulation in the arterial wall( Reference Suckling and Stange 51 ). Consistent with this study, we found that both lower and higher RV significantly lowered hepatic cholesterol content by 47 and 61 %, respectively, as compared to the HFD-alone group. Although we did not determine hepatic 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase activity and faecal bile acid levels in this study, the inhibition of hepatic HMG-CoA reductase activity or the increase of faecal bile acids excretion may be related to the plasma and hepatic cholesterol-lowering effect of RV. In our previous study, we demonstrated that RV led to decreased cholesterol level in plasma as well as the liver by suppressing the activity of hepatic HMG-CoA reductase, a rate-limiting enzyme for cholesterol synthesis, in apoE-deficient mice( Reference Do, Kwon and Kim 49 ). On the other hand, Zhu et al. ( Reference Zhu, Luo and Zin 52 ) reported that RV significantly lowered cholesterol levels in the serum and liver of HFD-fed rats and these effects resulted from the enhanced excretion of bile acids into faeces. Another possibility is an inhibition of pancreatic bile salt-dependent lipase by RV( Reference Sbarra, Ristorcelli and Petit-Thévenin 53 ). In rat pancreatic AR4-2J cells, RV inhibited the secretion and activity of bile salt-dependent lipase which is synthesised in pancreatic cells and involved in duodenal hydrolysis of lipid esters and absorption of non-esterified cholesterol.
Overall, our findings suggest that lower doses of RV may have more beneficial effects on adiposity, hepatic steatosis and hyperlipidaemia in HFD-fed mice compared to higher doses of RV. Currently, it is unclear why RV has a reverse dose–response effect, but it may explain why previous studies on the in vivo effects of RV have been equivocal. One possibility suggested in a recent review is that RV at high doses can exhibit pro-oxidant properties( Reference de la Lastra and Villegas 54 ). Oxidative stress closely correlates with fat accumulation in humans and mice( Reference Furukawa, Fujita and Shimabukuro 55 ) and reactive oxygen species lead to mitochondrial dysfunction associated with obesity and obesity-related metabolic disease( Reference Qatanani and Lazar 56 ). Considerable evidence supports that high doses of polyphenols can potentially cause adverse metabolic effects through pro-oxidative effects( Reference Martin and Appel 57 ). In fact, several recent papers indicate that high doses of RV in vivo can lead to adverse physiological effects. Dudley et al. ( Reference Dudley, Das and Mukherjee 58 ) reported that at lower doses (2·5 or 5 mg/kg body weight per d), RV protected against heart ischaemia by inducing a survival signal causing the up-regulation of anti-apoptotic and redox proteins, while at higher doses (>25 mg/kg body weight per d) RV potentiated a death signal causing the down-regulation of redox proteins and up-regulation of pro-apoptotic proteins. Consistently, RV protected the heart when administered at relatively low doses between 2·5 and 10 mg/kg body weight( Reference Das, Fraga and Das 59 ). In addition, high doses of trans-RV in hypercholesterolaemic rabbits have been reported to cause atherosclerotic lesions while lower doses of RV were found to be protective( Reference Wilson, Knight and Beitz 60 ).
In conclusion, the present findings suggest that dietary RV may be an effective anti-obesity agent and also protect against atherosclerosis when supplemented at low doses. RV decreases adiposity and hepatic steatosis in diet-induced obese mice and these effects appear to be mediated via regulation of lipid metabolism-related enzyme activity in the liver and adipose tissue, as summarised in Fig. 3. Low-dose RV was more beneficial for suppressing visceral adiposity and hepatic steatosis, along with protecting against dyslipidaemia in HFD-fed mice. In future studies, it will be important to establish whether varying doses of RV in humans consuming a HFD can provide any protection against hepatic steatosis, dyslipidaemia and visceral adipose tissue accumulation.

Fig. 3 Summary of the effects of resveratrol supplementation on lipid metabolism in the liver and adipose tissue.
![]() , Change in activity or concentration. FA, fatty acid; FAS, fatty acid synthase; G6PD, glucose-6-phosphate dehydrogenase; PAP, phosphatidate phosphohydrolase; Chol, cholesterol. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
, Change in activity or concentration. FA, fatty acid; FAS, fatty acid synthase; G6PD, glucose-6-phosphate dehydrogenase; PAP, phosphatidate phosphohydrolase; Chol, cholesterol. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Acknowledgements
The present study was supported by the National Research Foundation of Korea grant funded by the Korea government (no. 531-2006-1-C00064 and no. 2011-0000912). None of the authors had any conflict of interest. S.-J. C. carried out the experiments and U. J. J. wrote the original manuscript with S.-J. C. M.-S. C. performed substantial editing as well as the design of the experiment. All authors reviewed the literature and contributed to the final report.










