Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was first discovered and isolated in Wuhan, China, in December 2019. This is a new virus of zoonotic origin that has caused one of the largest known global pandemics [Reference Chih-Cheng1]. SARS-CoV-2 is a virus with a great capacity for infecting and transmitting. Nevertheless, not everyone responds in the same way to this virus. Most patients show symptoms as if they were suffering from a simple cold. However, other sufferers develop pneumonia and even toxic shock that leads to death [Reference Huang2, Reference Singh3]. There have been patients who have debuted with mucosal cutaneous manifestations, including enanthemas secondary to vasculitis, hives, varicella-like lesions, livedo reticularis, Covid toe, erythema multiforme and pityriasis rosea [Reference Elsaie, Youssef and Nada4–Reference Wollina6].
Certain pathogens use latency as an escape or evasion mechanism for the host's immune system. This is the case of the Herpesviridae family, such as herpes simplex type 1 and 2 (HSV-1 and HSV-2), varicella zoster virus (VZV), cytomegalovirus (CMV), human herpes virus 6, 7 (HHV 6, 7) and Epstein–Barr virus (EBV), although the cells in which they establish latency do vary. The most common entry point for human herpes viruses is the pharynx, although they can also enter through the genital or parenteral route. Once inside, they will use various mechanisms to colonise human cells and spread, including the use of receptors and co-receptors on the surface of human cells. After contact, the virus fuses its lipid envelope with the host cell membrane and releases its nucleocapsid together with the integument proteins into the cytosol. From here, it will introduce its viral-DNA to the nucleus of the host cell, replicating rapidly and generating infection. After the initial infection, all herpes viruses remain in a state of latency, in different host cells, from where they can be later reactivated [Reference Madavaraju7]. Here, we evaluate three cases of herpes infection, in which HSV-1 and VZV were held responsible. Although the host has a strong and effective immune system, the virus remains quartered in Gasser's ganglion (HSV-1) or in the ganglia of the nerve roots (VZV), without generating symptoms or pathology. In a latently infected neuron, virus-specific proteins are not produced, and as a result, the host's immune system does not identify the presence of the virus and does not target the latently infected neuron for destruction. The host may suffer from an immunodeficiency, triggered by any of a large number of reasons, including stress, insomnia, insolation, malnutrition, taking immunosuppressive drugs, pregnancy, ageing, infections by other viruses and debilitating diseases. When this occurs, the herpes virus appears on the skin, either: in the facial area (HSV-1), such as lips, nose, cheekbones and even in the eyes, which can cause herpetic keratitis with the risk of blindness, or it appears in the cervical, dorsal or lumbar areas (VZV) causing a herpetic neuropathy [Reference Inbaraj8, Reference Kubota9]. The lesions appear as raised erythematous plaques upon which maculo-papulous vesicles settle, spreading through the affected dermatome, and usually accompanied by pain, itching and burning in the area. Herpes virus infections can occur as primary or nosocomial pathogens, but clinical manifestations are most commonly a re-activation of a latent viral infection [Reference Kushawaha, Mobarakai and Tolia10].
It is common knowledge that lifestyles with physical activity, and social, family and cultural contacts constitute healthy habits that improve immune defences and the mental and emotional state [Reference Filgueira11]. The confinement of families in their homes, towns, cities and even countries, isolated from other relatives, friends and neighbours due to the coronavirus disease 2019 (COVID-19) pandemic has, for many people, presented a very stressful life event, triggering never-before-lived experiences that have threatened the subject's health and well-being. The wide-ranging consequences of these restrictive measures include a sedentary lifestyle, obesity, hypertension, increases in cortisol and immunosuppression [Reference Wollenstein-Betech12].
The three cases presented herein show that there is a close relationship between the appearance of herpes infections and the COVID-19 pandemic situation, and that a secondary immunodeficiency state is generated, either due to the SARS-CoV-2 infection or due to the stress generated by the pandemic itself.
Presentation of case 1: herpes varicella zoster infection
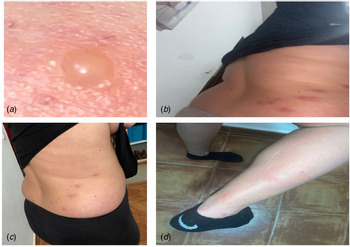
We report the case of a 62-year-old Spanish man, subjected to the first Spanish total lockdown from 14th March 2020 to 21st June 2020. On 11th May 2020, the patient presented elevated erythematous lesions with vesicles on his left side compatible with a varicella zoster infection (Fig. 1), having been diagnosed and evaluated by his Primary Care Centre. General malaise with fever and diarrhoea accompanied the dermatological lesions. The patient was quickly treated, first 24–48 h, with acyclovir for 7 days, oral and topically, as well as antipyretics for the fever. On questioning, he emphasised that he had previously been in good health, had received all necessary vaccinations and that he had not previously suffered from any fever, that he was not a drug user, and that he drank no alcoholic beverages, and had not travelled. He also stated that he had been grieving the loss of a family member to COVID-19. In short, a patient with no medical history of interest except for (a) benign prostate hypertrophy, compatible with his age, under treatment with silodyx, a specific alpha-adrenergic antagonist, and (b) his low mood. The evolution of the patient was good. Nasopharyngeal and blood samples obtained from the patient revealed a negative reverse transcriptase polymerase chain reaction (RT-PCR) and negative antibody [immunoglobulin M (IgM) and IgG] test for SARS-CoV-2.
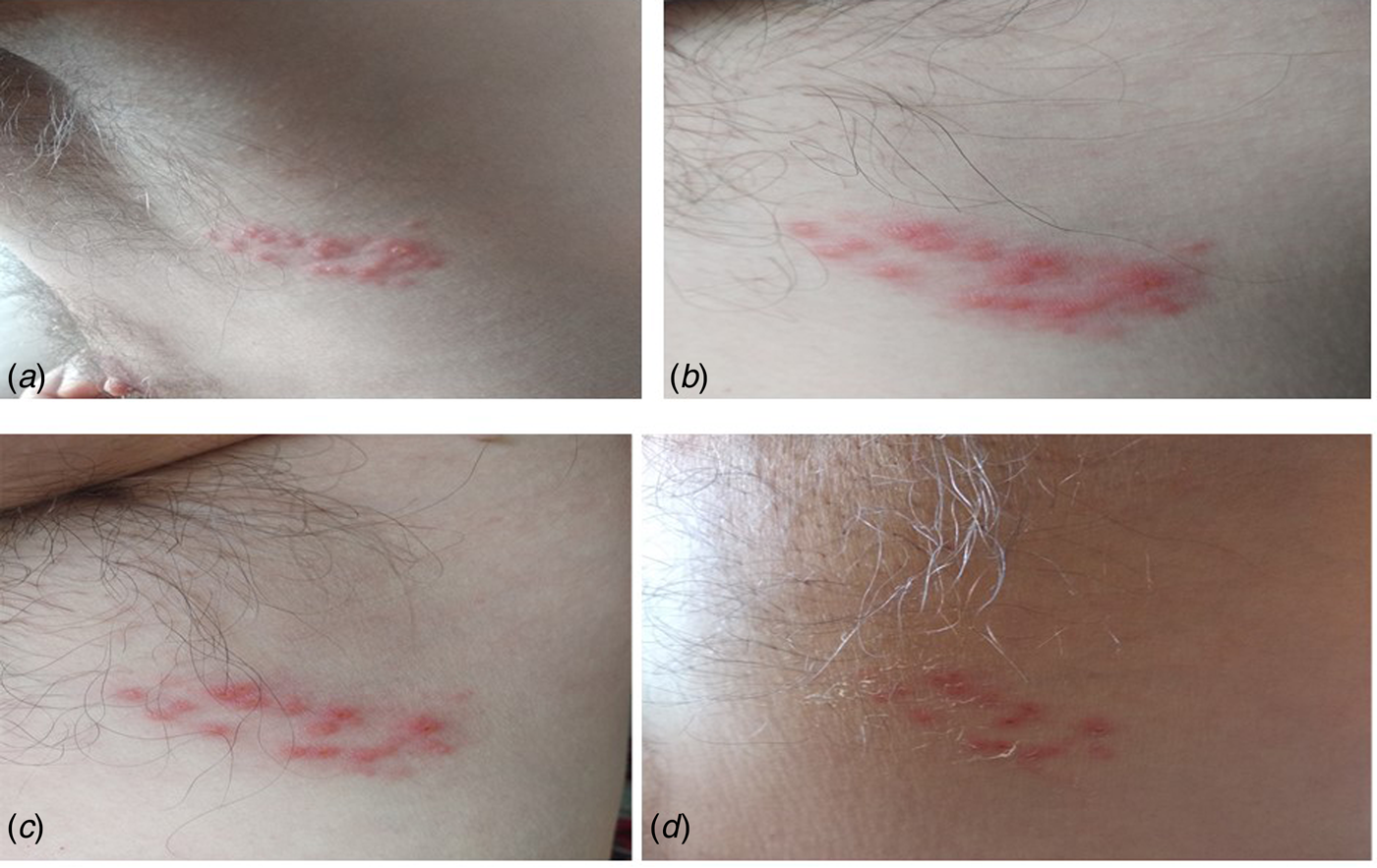
Fig. 1. Case 1. (A) Onset of lesions with raised vesicles over erythematous areas; (B and C) evolutionary periods with favourable remission of the herpetic infection and (D) advanced period of remission with vesicular drying and peeling of the skin. Negative RT-PCR for SARS-CoV-2.
Presentation of case 2: herpes simplex 1 infection
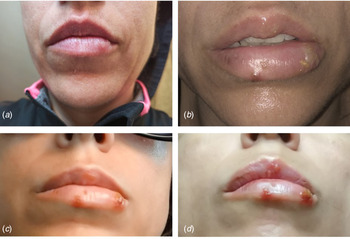
We report the case of a 40-year-old Spanish woman, subjected to the extension of the third state of alarm in Spanish from 9th November 2020 until 9th May 2021. On 13th December 2020, the patient reported a prodromal phase of 2 days, with a burning sensation in her lower lip and hyperesthesia in the area around the mouth. A large vesicle subsequently appeared on her lower lip together with other smaller vesicles that broke at different times, which suppurated and left ulcerations and scabs that remitted after 10 days of evolution (Fig. 2). This clinical state was accompanied by fever and general malaise. She was diagnosed with herpes simplex 1 infection and treated symptomatically by her Primary Care Centre with paracetamol, antihistamines and topical aloe-vera, with a good evolution. The patient's personal history revealed no data of interest, except for the situation of social isolation due to the pandemic. Nasopharyngeal and blood samples obtained from the patient revealed a negative RT-PCR and negative antibody (IgM and IgG) test for SARS-CoV-2.
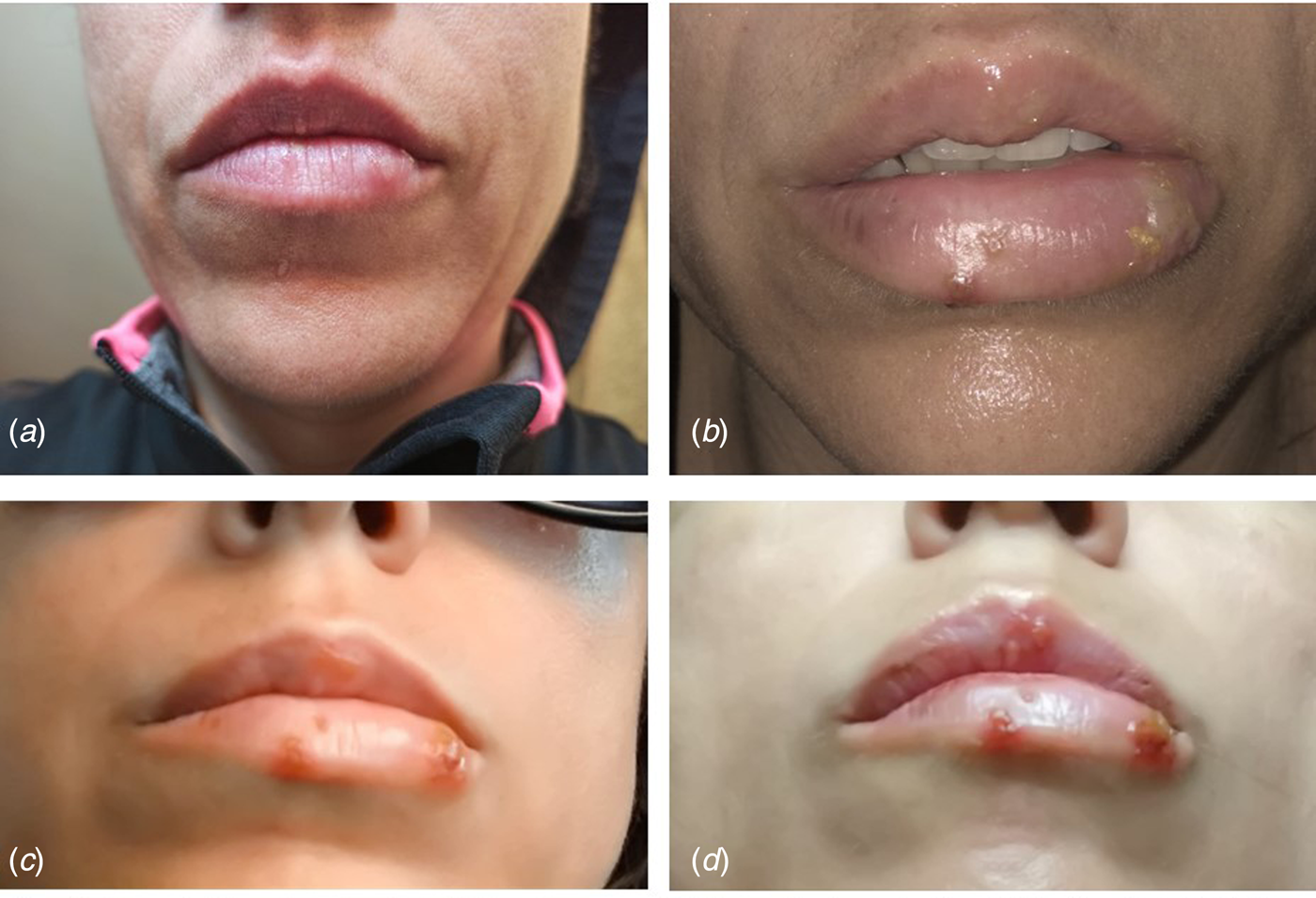
Fig. 2. Case 2. (A) Prodormal period with burning area and itching on the lower lip; (B and C) florid period of infection with increased number of vesicles on the lips and (D) referral period. Negative RT-PCR for SARS-CoV-2.
Presentation of case 3: herpes varicella zoster infection with subsequent positive diagnosis of SARS-CoV-2
A 25-year-old woman, suffering from a fever of 38 °C and the appearance of very itchy vesicular lesions on her right-hand side lumbar area, came to the emergency room of her Primary Care Centre, during the month of February 2021, which coincided with the third wave of COVID-19 in Andalusia (Spain). She presented vesicular injuries that later spread to her arms and legs (Fig. 3). She was initially diagnosed with varicella zoster infection. The patient was discharged with a treatment based on paracetamol and topical calamine powders for the itchy vesicles. After a week of evolution, she presented a worsening of her general condition, with a fever of up to 40 °C with chills, an unproductive dry cough, dyspnoea, fatigue and leg pain. She was treated again, this time as an in-patient in the hospital, and an RT-PCR was performed against the SARS-CoV-2 virus, through which she was diagnosed as being positive.
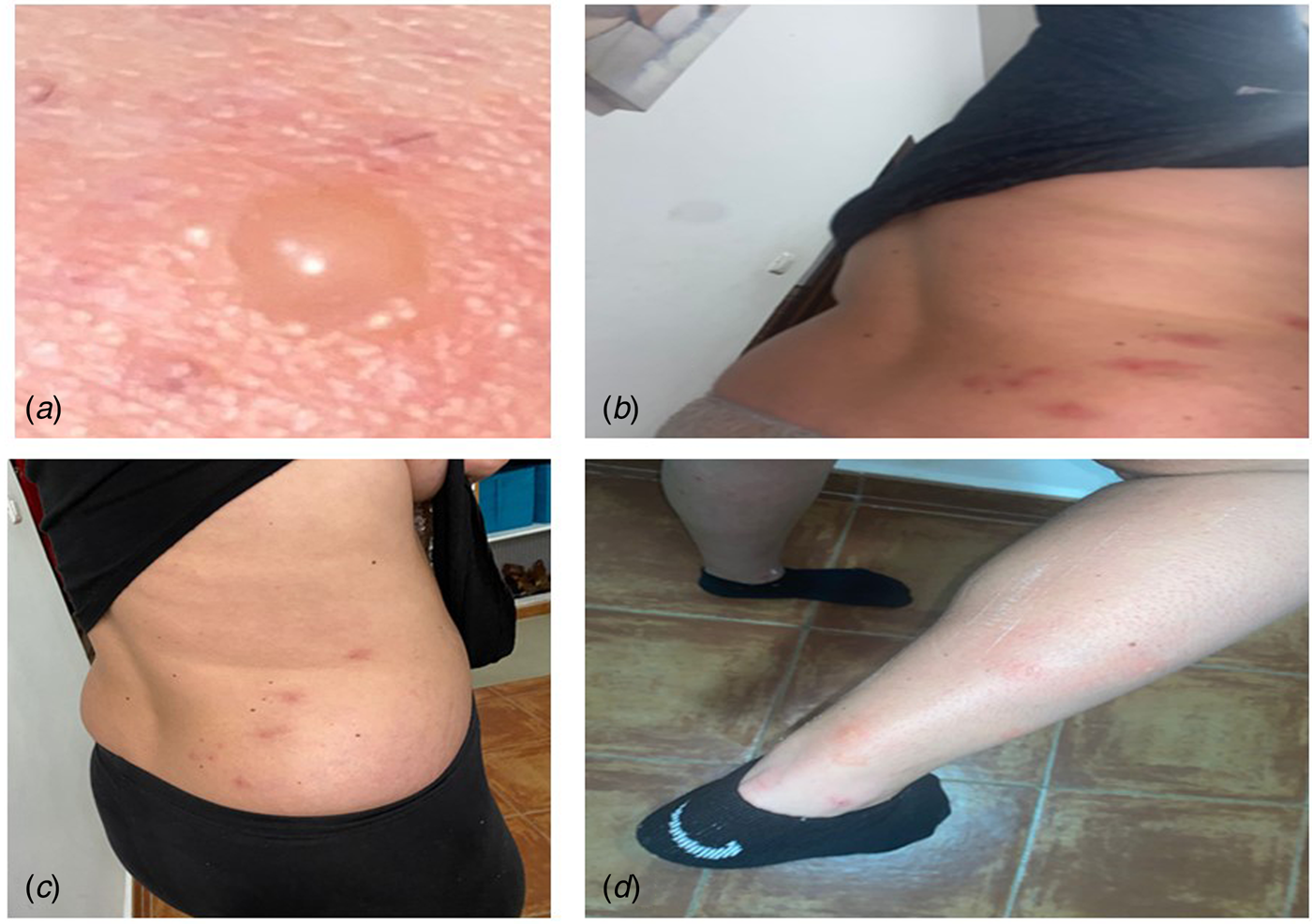
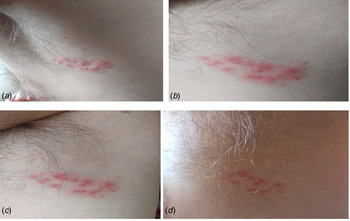
Fig. 3. Case 3. (A) Close-up of herpes varicella zoster lesion; (B and C) initiation of vesicles in the right lumbar dorsum and (D) posterior extension of the vesicles to the right leg. Positive RT-PCR for SARS-CoV-2.
Discussion
The appearance of the herpes virus infection in times of the COVID-19 pandemic in patients, with or without respiratory symptoms, should make us aware of the possibility of having an underlying SARS-CoV-2 infection [Reference Elsaie, Youssef and Nada4]. The SARS-CoV-2 virus is a new contagious beta-coronavirus that is transmitted person to person mainly via the air. Initially, it affects the respiratory system by entering the host's respiratory epithelial cells, through the S protein (transmembrane spike glycoprotein) of its outer capsule, using the ACE2 receptor [Reference Hoffmann13]. The infection is established in the pneumocytes, causing the activation of innate immunity with the release of type 1 interferons (INF-alpha and INF-beta) from the infected cells. Furthermore, molecular patterns associated with the pathogen or damage, known as PAMP and DAMP respectively are generated and recognised, which can lead to the activation of alveolar macrophages (phenotype M1) responsible for the release of pro-inflammatory cytokines such as interleukin (IL)-1 beta, tumour necrosis factor-alpha, IL-6, IL-8 and IL-12 [Reference Li14]. IL-12, in turn, activates natural killer (NK) cells and the specific or adaptive immune response with the activation of T and B lymphocytes. Up to this point, we would be facing a standard viral defence [Reference Abbas, Lichtman and Pillais15]. However, SARS-CoV-2 has a series of evasion mechanisms that allow it to circumvent our immune system, thereby leaving the host vulnerable and thus facilitating replication of the virus and the increase in viral load. From among the evasion mechanisms used by SARS-CoV-2 we highlight (a) alteration of the synthesis and functionality of INF type 1 (INF-alpha and beta) and 2 (INF-gamma). This allows the virus to replicate in host cells without opposition or without an effective antiviral state [Reference Huang2, Reference Acharya, Liu and Gack16]; (b) cytokine storm or excessive activation of M1 macrophages with an inordinate amount of pro-inflammatory cytokines released into the serum, whose synergistic effects would be responsible for the severity of patients infected by SARS-CoV-2 [Reference Li14]. Cytokines released in large quantities cause fever, increased acute-phase proteins, increased adhesion molecules in the vascular endothelium, edema, activation of the coagulation system with disseminated intravascular coagulation, increased cell catabolism and decreased cardiac output with multi-organ damage [Reference Abbas, Lichtman and Pillais15, Reference Tay17]. This altered immune response facilitates the replication of the virus and the increase in the viral load, by means of causing the NK cells and CD8+ lymph T cells to become exhausted and hence the coronavirus cannot be eliminated [Reference Zheng18]. In the same way as occurs in other states of hyperactivation of the immune system, such as burn patients, polytraumatised patients and head trauma patients, the increase in adhesion molecules could generate a state of immunodeficiency of T and B lymphocytes which would remain attached to the endothelium of the blood vessels and therefore unable to enter the site where they should perform their function [Reference Maldonado19, Reference Cao20]. In addition, the SARS-CoV-2 infection produces a reduction in the percentages of monocytes, eosinophils and basophils [Reference Huang2].
On the other hand, the different confinements of people, in their homes, towns, provinces and even countries, carried out during the COVID-19 pandemic has led not only to an increase in social disconnection, but also in the levels of anxiety, stress and depression in the population [Reference Ozamiz-Etxebarria21]. This has provoked an unprecedented situation in the Spanish population that has generated increases in the levels of cortisol, catecholamines and certain opiates, substances which are generally immunosuppressive and involve several pathways, including lymphocytopenia and hypogammaglobulinaemia [Reference Eisenbergera22].
Over recent decades, the interaction between the neuroendocrine and immune systems has frequently been suspected. The integration between the two systems is based on a bidirectional communication, which in immunobiochemical and molecular terms, implies having intercellular signals and common recognition systems. In this respect, it is currently recognised and accepted that many neuroendocrine signals are produced by immunocompetent cells, and that these same cells also express specific receptors for these neuroendocrine signals [Reference Irwin23–Reference Maldonado26]. On the contrary, typical cytokines produced by immunocompetent cells, as well as the receptor molecules for these cytokines, are produced and expressed by neuroendocrine cells [Reference Raony27]. This neuroendocrine–immune interaction is currently of recognised and growing importance and is considered to play a fundamental role in the generation of diseases [Reference Ziemssen and Kern28]. It has been observed how disturbances in the interactions among the nervous, immune and endocrine systems are implicated in various diseases such as an increased number of infections, decreased responses to vaccines, the delayed healing of wounds and a higher incidence of oncological diseases and autoimmune diseases [Reference Ziemssen29]. Both, the stress generated during the pandemic period and the SARS-CoV-2 invasion itself are generative elements of immunodeficiency in humans, a situation that could be exploited by the herpes virus to reactivate and infect the host [Reference Raony27]. Hence, it is no surprise that the herpes virus infection may be the first manifestation, as if it were a prodromal period, prior to the appearance of symptoms of the SARS-CoV-2 infection. This is the series of events in our third clinical case, that of the 25-year-old woman, whose SARS CoV-2 infection initially debuted with disseminated herpes varicella zoster (Fig. 3).
A previous case report by Elsaie et al. [Reference Elsaie, Youssef and Nada4] suggested that herpes varicella zoster might be an indicator for latent COVID-19 infection; the authors provide two clinical cases of patients with SARS-CoV-2 infection who initially presented herpes varicella zoster lesions. Jimenez-Cauhe et al. [Reference Jimenez-Cauhe30] have found erythema multiforme-like lesions in the skin of four hospitalised patients with the COVID-19 infection. It remains unclear, however, whether they were specific lesions of SARS-CoV-2, or of other virus infections, or the result of the drugs administered during the admission of the patients in hospital. Ferreira et al. [Reference Ferreira31] describe a case of a 39-year-old man, who was immunocompetent with SARS-CoV-2 and co-infected with herpes varicella zoster. The authors argue that SARS-CoV-2 probably induced a retroactive reactivation of the herpes virus. Tartari F et al. [Reference Tartari32] reported four more cases of herpes virus infections in patients with positive SARS-CoV-2. The authors explain that all their patients showed a decrease in peripheral blood of the T lymphocyte subpopulations, and specifically a decrease in CD3+ and CD8+ T lymphocytes prior to the onset of herpes varicella zoster. Furthermore, Balc'h et al. [Reference Balc'h33] found 18 SARS-CoV-2-positive patients with lymphopenia and reactivation of herpes simplex virus and cytomegalovirus; these authors consider that the SARS-CoV-2 infection may actually constitute the risk factor for reactivation.
In summary, we show three clinical cases of the herpes virus infection, two of which yield negative results on RT-PCR for SARS-CoV-2 and one testing positive. All these cases occurred during the COVID-19 pandemic period on Spanish territory. The lesions presented by our patients and those in other aforementioned cases, support the hypothesis that a herpes infection can manifest itself prior to suffering from a SARS-CoV-2 infection and, although it needs to be tested in larger cohorts of patients, performing a PCR test on subjects with active herpes virus infections could increase the number of cases of SARS-CoV-2 detected early, precisely at the stage when this deadly virus is most infectious.
Acknowledgements
This study was supported by Seville University (Immunology area), Department of Medical Biochemistry, Molecular Biology and Immunology; also, by the Ministry of Economy, Industry and Competitiveness (MINECO 2017), reference: BFU2017-85832-R.
Author contributions
M. D. Maldonado conducted the literature review, interpreted the immunological and clinical data of the patients and she was the major contributor to the manuscript. J. Romero-Aibar participated in the care and analytical follow-up of the patients as a senior laboratory technician and helped in revising the manuscript. M. A. Perez-San-Gregorio performed the analysis of the mental state of the patients and directed the review of the psychological literature.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
Written informed consent was obtained from the patients for publication, of this cases report included in this article, according to the specifications established by the Ethics Committee of the University of Seville for the publication of clinical cases.
Data availability statement
Data supporting the findings of this study are available at SAS (Andalusian Health Service Spain). Restrictions apply to the availability of these data, which were used under license for this study. The data have been made available, to the authors, with the permission of affected patients.