INTRODUCTION
Malaria during pregnancy is still a major public health problem in sub-Saharan Africa. Indeed in this region, approximately 25 million pregnant women are at risk of Plasmodium falciparum infection every year, and about 25% of women carry placental P. falciparum infection at the time of delivery (Desai et al. Reference Desai, ter Kuile, Nosten, McGready, Asamoa, Brabin and Newman2007). Plasmodium falciparum infection in pregnancy may result in parasite sequestration in maternal placental vascular space (Steketee et al. Reference Steketee, Nahlen, Parise and Menendez2001), this phenomenon often causes adverse outcomes such as intrauterine growth restriction, preterm delivery, low birthweight (LBW), stillbirth, early neonatal death and maternal anaemia (Steketee et al. Reference Steketee, Nahlen, Parise and Menendez2001; Desai et al. Reference Desai, ter Kuile, Nosten, McGready, Asamoa, Brabin and Newman2007). The proportion of severe anaemia during pregnancy attributable to malaria is estimated to be 26%, irrespective of maternal gravidities (Desai et al. Reference Desai, ter Kuile, Nosten, McGready, Asamoa, Brabin and Newman2007). In areas of high malaria transmission in Africa, the risk of LBW approximately doubles if women have placental malaria (Guyatt and Snow, Reference Guyatt and Snow2004).
The World Health Organization (WHO) recommends the administration of intermittent preventive treatment with sulfadoxine-pyrimethamine (IPTp-SP), the use of insecticide-treated nets and the effective management of clinical cases as relevant strategies to reduce the burden of malaria and improve birth outcomes (WHO, 2004). Recently, the WHO has recommended that SP should be provided at each scheduled focused antenatal care (ANC) visit in the second and third trimesters (WHO, 2012).
Burkina Faso, a poor country located in West Africa is holoendemic for malaria. Many studies have reported placental P. falciparum malaria with a prevalence ranging from 15·2 to 22·7% in rural areas (Sirima et al. Reference Sirima, Cotte, Konaté, Moran, Asamoa, Bougouma, Diarra, Ouédraogo, Parise and Newman2006; Gies et al. Reference Gies, Coulibaly, Ouattara, Ky, Brabin and D'Alessandro2008, Reference Gies, Coulibaly, Ouattara and D'Alessandro2009; Tiono et al. Reference Tiono, Ouedraogo, Bougouma, Diarra, Konaté, Nébié and Sirima2009) and from 4·7 to 13% in urban areas (Molez et al. Reference Molez, Bosseno, Traore, Carnevale and Gazin1992; Gazin et al. Reference Gazin, Compaoré, Hutin and Molez1994; Bamba et al. Reference Bamba, Séré, Nikiéma, Halidou, Thiéba, Dao and Guiguemdé2013). However, in the periurban settings, there are few and old data on the prevalence of placental malaria including Bobo-Dioulasso, the country's second largest city (Molez et al. Reference Molez, Bosseno, Traore, Carnevale and Gazin1992; Gazin et al. Reference Gazin, Compaoré, Hutin and Molez1994; Bamba et al. Reference Bamba, Séré, Nikiéma, Halidou, Thiéba, Dao and Guiguemdé2013). Furthermore, the effects of placental malaria on maternal and newborn outcomes have been poorly documented in this town and the present study sought to fill this knowledge gap.
METHODS
Study site
This study was conducted in Bobo-Dioulasso, a town located 365 km, South-West of Ouagadougou, the capital city of Burkina Faso. There are an estimated to 800 000 inhabitants of Bobo-Dioulasso with farming and trading as main economic activities. The site is an area of high malaria transmission season from May to November. The average entomological inoculation rate is about 63 infectious bites per person per year in the periurban area of Bobo-Dioulasso (Diabaté, Reference Diabaté2003).
Study design
We conducted a cross-sectional study from September to December 2010 in the primary health facilities of Kua and Lafiabougou both located in the periurban area of Bobo-Dioulasso.
Participants
Study participants were women attending the two primary health facilities for delivery. Women with alive singleton births were included in the study; exclusion criteria were women with pathological pregnancies that could affect the placenta such as retro placental hematoma placenta previa.
Collection of personal data and blood samples
Information on maternal baseline (age, education, parity), utilization of health services during pregnancy such as the number of ANC visits and use of malaria prevention (use of IPTp-SP and bed net) was collected from participating women using a standardized questionnaire. Data on gestational age was not collected due to high likelihood of inaccuracy among illiterate mothers. Finger prick blood from mothers was used for blood smear and haemoglobin measurement. After birth, the umbilical cord was cut and clamped at about 3 cm from newborn abdomen. Umbilical cord blood was collected by releasing the clamp and compression and was used for parasitological studies. The newborns were dried and weighed. After placental expulsion, blood was collected from the maternal face and used to measure parasite density.
Laboratory methods
Thin and thick blood smears of maternal peripheral or cord blood and thin blood smears of placental blood were stained with 10% Giemsa dye and examined under oil immersion for parasites. Parasite density was determined by counting asexual forms of the parasite per 200 leukocytes and calculating parasites µL−1 by assuming an average of 8000 leukocytes µL−1 of blood. A slide was considered negative if no parasite was found after counting 500 leukocytes. Parasitaemia was classified as low (<500 parasite µL−1 of blood), moderate (501–5000 parasites µL−1 of blood) and high (>5000 parasites µL−1 of blood) (Tonga et al. Reference Tonga, Kimbi, Anchang-Kimbi, Nyabeyeu, Bissemou and Lehman2013). All the slides were double-checked blindly and for discrepant results a third consensus reading was performed.
Haemoglobin concentration was measured using a haemoglobinometer (HaemoCue AB, Angelhom, Sweden). Anaemia was defined as a haemoglobin concentration lower than 11·0 g dL−1.
Sample size
The expected number of pregnancies in the two primary health facilities was 2400 in 2010. The sample size calculation (n = 320) was based on a prevalence of placental P. falciparum parasitaemia of 4% (Bamba et al. Reference Bamba, Séré, Nikiéma, Halidou, Thiéba, Dao and Guiguemdé2013) with a relative precision of 2% and alpha of 5%.
Data entry statistical analyses
Data were double entered in Excel 2013 and analyses performed using STATA 12 (Stata Corp., College Station, Texas, USA).
Utilization of IPTp-SP was dichotomized into <2 doses vs ≥2 doses. LBW was defined for <2500 g. Statistical calculations relied purposely on the Log10 of parasite density.
Proportions for categorical variables were compared with Pearson-chi2 test. Means comparisons between groups were done by the Student's t-test or One-way analysis of variance (ANOVA) test. When at least one of the group means was different from the other group means by using One-way ANOVA test, then a Tukey post hoc test was used to determine which groups differed from each other. Correlation between continuous variables was measured with Pearson correlation coefficient. We used logistic regression models to identify categorical variables associated with placental P. falciparum parasitaemia. Associations between placental parasitaemia and categorical variables (anaemia and LBW) and continuous variables (haemoglobin concentration and birthweight) were investigated by logistic and linear regression, respectively. Multivariable analyses were built using forward stepwise regression models, with an inclusion criterion of P < 0·2. Statistical significance was set for P < 0·05.
Ethical considerations
This study was approved by the National Ethics Committee for Health Research, Ouagadougou Burkina Faso (NECHR, Ouagadougou, BF No 2010-054). A written informed consent was obtained from all study participants prior to their enrolment. For illiterate delivering women, the informed consent discussion process was witnessed by an impartial individual, and the informed consent form was endorsed with a thumbprint. Women with moderate anaemia were treated with folic acid supplementation; those who had placental P. falciparum malaria received oral quinine at a dose of 24 mg day−1 for 7 days as recommended by the national malaria control program in Burkina Faso.
RESULTS
Description of study participant
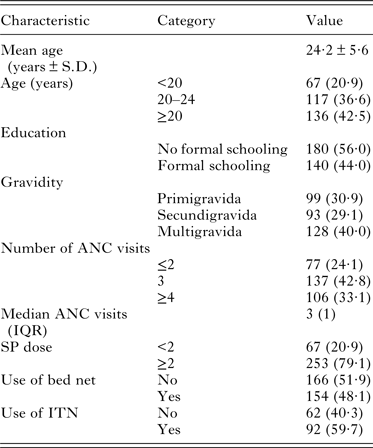
A total of 320 mother-newborn pairs were included into the study. The mean maternal age was 24·2 ± 5·6 years. The median number of ANC visits was 3 (ranged from 0 to 5) and over 33% of mothers attended at least 4 ANC visits. A total of 8 participants (2·5%) did not receive any dose of IPTp-SP during pregnancy, while 79% of participants received ≥2 doses (Table 1). About 48% of women reported sleeping under a bed net and among them only 59·7% used insecticide-treated net (ITN).
Table 1. Baseline characteristics of the study population
Maternal and placental parasitaemia
Overall, malaria parasite was found in 55 (17·2%) peripheral blood films and 29 (9·1%) placental blood films. Plasmodium falciparum was the sole species found in all cases. Both trophozoite and schizont forms of P. falciparum were found in placental blood samples. Among the 55 peripheral blood samples, both forms of P. falciparum were found in only one sample. Of the 320 women, 26 (8·1%) women had concurrent peripheral and placental parasitaemia, 29 (9·0%) women had peripheral infections only, while 3 (0·9%) had placental infection only. The arithmetic mean parasite density in placental blood (4·5 ± 0·8 µL−1) was significantly higher than that in peripheral (3·7 ± 0·7 µL−1) (P < 0·001). The proportion of placenta with high parasite density (>5000 µL−1) is higher compared with that of peripheral blood with high parasite density (P = 0·01). Peripheral blood parasite density correlated well with placental blood parasite density (r = 0·49, P = 0·01). The Tukey post-hoc test revealed that mean parasite density in peripheral blood was statistically significantly lower in the multigravida group compared with the primigravida group (−0·5 ± 0·2 parasite µL−1, P = 0·04). However, there were no statistically significant differences between the multigravida and secundigravida groups (−0·2 ± 0·2 parasite µL−1, P = 0·6), or the secundigravida and primigravida groups (−0·4 ± 0·2 parasite µL−1, P = 0·3). The mean parasite density in placental blood was not statistically significant different between gravidity groups (F (2, 26) = 1·75, P = 0·2).
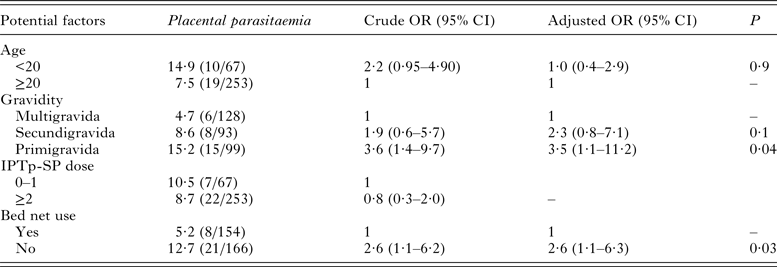
Overall, the univariable regression analysis showed that primigravida (OR: 3·6; 95% CI (1·4–9·7)) and women who did not sleep under bed net (OR: 2·6; 95% CI (1·1–6·2)) were at higher odds of P. falciparum placental parasitaemia (Table 2). The use of IPTp-SP was not associated with the prevalence of placental malaria infection. These potential factors were further subjected to multivariable logistic regression. Finally, primigravida and women who did not sleep under bed net had 3·5- and 2·6-folds increased odds of placental malaria infection, respectively.
Table 2. Risk factors associated with placental parasitaemia among delivering women in Bobo-Dioulasso
Effect of placental P. falciparum parasitaemia on maternal outcomes
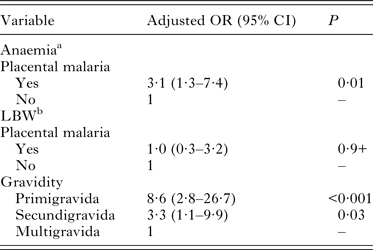
The proportion of anaemia among all women was 48·4%. The prevalence of anaemia was significantly higher in women with placental malaria parasitaemia (72·4%) than in uninfected women (46·1%). Placental malaria parasitaemia was associated with a 3·1-folds increase odds of maternal anaemia (aOR: 3·1; 95% CI (1·3–7·4)) (Table 3). The mean haemoglobin level among study participant was 10·9 ± 1·7 g dL−1 and infected women had 0·8 g dL−1 lower than uninfected women (P = 0·01) (Table 4).
Table 3. Effect of placental malaria on maternal anaemia and low birthweight in multivariable logistic regression analysis
a Regression analysis adjusted by age, gravidity, use of IPTp-SP, and use of bed net.
b Regression analysis adjusted by newborn's gender.
Table 4. Effect of placental malaria on maternal haemoglobin concentration and birthweight in multivariable linear regression analysis

a Regression analysis adjusted by age, gravidity, use of IPTp-SP, and use of bed net.
b Regression analysis adjusted by age, gravidity, use of IPTp-SP, use of bed net and newborn's gender.
Effect of placental P. falciparum parasitaemia on newborn outcomes
The overall prevalence of malaria parasite infection in newborns was 0·9% (3/320). The prevalence of congenital malaria parasitaemia was 10·3% (3/29) among infants born to mothers with placental malaria parasitaemia. The three newborns were infected by the trophozoite forms of P. falciparum with parasite density ranging from 200 to 1120 µL−1. The mothers of the newborns had both high peripheral and placental malaria parasitaemia as shown in Table 5. Furthermore, they have benefited from at least 2 doses of IPTp-SP and two of them were primigravida.
Table 5. Distribution of the 3 cases of congenital malaria according to their mothers’ age, obstetric history and malaria infection

Overall, the mean singleton live-born birth weight was 2917 ± 426 g (Table 4). A total of 37 neonates (11·6%) had LBW. The prevalence of LBW was higher among babies born from women with placental parasitaemia (13·8%) compared with those without (11·3%) (P > 0·05). Factors associated with LBW in multivariable analysis were primigravidity (aOR = 8·6; 95% CI (2·8–26·7)) and secundigravidity (aOR = 3·3; 95% CI (1·1–9·9)) (Table 3). Neonates born from mothers with placental malaria parasitaemia had a significant mean birthweight reduction of 200 g compared with those born from mothers without placental malaria parasitaemia (Table 4). In addition, there was a significant negative correlation between birthweight and maternal parasitaemia density (r = 0·32; P = 0·02). In contrast, there was no correlation between placental malaria parasitaemia density and birthweight of the newborns (r = 0·08; P = 0·7).
DISCUSSION
The aim of this study was to determine the prevalence and the impact of placental malaria parasitaemia on maternal anaemia, congenital malaria infection and birthweight in periurban area of Bobo-Dioulasso.
The overall prevalence of placental malaria parasitaemia (9·1%) was higher than the reported prevalence (4·7%) in urban area of Bobo-Dioulasso by (Bamba et al. Reference Bamba, Séré, Nikiéma, Halidou, Thiéba, Dao and Guiguemdé2013). This could be explained by the location of our study sites in the periurban area of Bobo-Dioulasso where the transmission of malaria is thought to be higher than in the urban part of the town (Diabaté, Reference Diabaté2003). This trend is consistent with previous reports in this town where the prevalence in periurban area of the town was 29% in 1984 and 18% in 1991 compared with those of the urban part in 1984 (9%) and in 1991 (13%) (Molez et al. Reference Molez, Bosseno, Traore, Carnevale and Gazin1992; Gazin et al. Reference Gazin, Compaoré, Hutin and Molez1994). As can be seen, there was a trend of decrease of the prevalence of placental infection over the years probably due to the increase in malaria prevention measures coverage. Our findings are lower than those reported in rural area of Burkina Faso ranging from 15·9 to 22·7% (Sirima et al. Reference Sirima, Cotte, Konaté, Moran, Asamoa, Bougouma, Diarra, Ouédraogo, Parise and Newman2006; Gies et al. Reference Gies, Coulibaly, Ouattara, Ky, Brabin and D'Alessandro2008, Reference Gies, Coulibaly, Ouattara and D'Alessandro2009; Tiono et al. Reference Tiono, Ouedraogo, Bougouma, Diarra, Konaté, Nébié and Sirima2009). The higher observed rates in rural area could be explained by the high transmission level of malaria in rural area of Burkina Faso (Diabaté, Reference Diabaté2003). The prevalence of placental malaria parasitaemia in urban and periurban settings in Africa varied from 1·6 to 69·6% (Morgan, Reference Morgan1994; Ndao et al. Reference Ndao, Ndiaye, Gaye and Le Hesran2003; Sarr et al. Reference Sarr, Marrama, Gaye, Dangou, Niang, Mercereau-Puijalon, Lehesran and Jambou2006; Namusoke et al. Reference Namusoke, Rasti, Kironde, Wahlgren and Mirembe2010; Famanta et al. Reference Famanta, Diakite, Diawara, Diakité, Doumbia, Traoré, Konaté, Doumbia, Keita, Thiéro, Traoré, Doumbia and Tounkara2011; Ezebialu et al. Reference Ezebialu, Eke, Ezeagwuna, Nwachukwu, Ifediata and Ezebialu2012; Bassey et al. Reference Bassey, Nyengidiki and John2015). The wide ranges in reported prevalence of placental malaria infection may be due to multiple factors. First, one factor is the method of diagnosis. Indeed most of the studies (Sarr et al. Reference Sarr, Marrama, Gaye, Dangou, Niang, Mercereau-Puijalon, Lehesran and Jambou2006; Namusoke et al. Reference Namusoke, Rasti, Kironde, Wahlgren and Mirembe2010; Ezebialu et al. Reference Ezebialu, Eke, Ezeagwuna, Nwachukwu, Ifediata and Ezebialu2012) had used placental histology, which is recognized as the gold standard test and more sensitive than microscopy (Kattenberg et al. Reference Kattenberg, Ochodo, Boer, Schallig, Mens and Leeflang2011). Second, other factors that may explain this variation include intensity of transmission, study population characteristics (age, parity, HIV status), use of preventive measures (IPTp-SP, ITNs) (Mokuolu et al. Reference Mokuolu, Falade, Orogade, Okafor, Adedoyin, Oguonu, Dada-Adegbola, Oguntayo, Ernest, Hamer and Callahan2009).
We observed 32 discordant results between placental and peripheral parasitaemia and this is consistent with previous reports (Matteelli et al. Reference Matteelli, Donato, Muchi, Leopardi, Astroli and Carosi1994; Ezebialu et al. Reference Ezebialu, Eke, Ezeagwuna, Nwachukwu, Ifediata and Ezebialu2012). Placental parasitaemia without peripheral parasitaemia may be a feature in women who have previously been treated with clearance of peripheral parasite whereas peripheral parasitaemia without placental infection may occur in early malaria infection, especially if parasitaemia is low (Ezebialu et al. Reference Ezebialu, Eke, Ezeagwuna, Nwachukwu, Ifediata and Ezebialu2012).
Malaria infection with parasite density higher than 5000 parasites µL−1 was significantly higher in placental blood than in peripheral blood. Parasites variant antigens expressed on the surface of infected erythrocytes results in a preferential sequestration of parasitized red blood cells in the placenta, with subsequent increase on malaria parasite density even in the absence of peripheral blood parasitaemia (Staalsoe et al. Reference Staalsoe, Megnekou, Fievét, Ricke, Zornig, Leke, Taylor, Deloron and Hviid2001; Brabin et al. Reference Brabin, Romagosa, Abdelgalil, Menéndez, Verhoeff, McGready, Fletcher, Owens, D'Alessandro, Nosten, Fischer and Ordi2004).
This study demonstrated that primigravida are 3·5 times more likely to have placental malaria than multigravida. Pregnancy is associated with a decrease in immunity, which is more pronounced in primigravida than in multigravida. Indeed, Primigravida express specific placental receptors that facilitate binding of parasitize erythrocytes to the placental tissue, but this is less likely in the multigravida due to acquisition of specific antibodies that prevent such binding from occurring (Staalsoe et al. Reference Staalsoe, Megnekou, Fievét, Ricke, Zornig, Leke, Taylor, Deloron and Hviid2001; Brabin et al. Reference Brabin, Romagosa, Abdelgalil, Menéndez, Verhoeff, McGready, Fletcher, Owens, D'Alessandro, Nosten, Fischer and Ordi2004).
The use of bed nets was associated with reduced odds of placental malaria parasitaemia despite a low coverage of ITNs. Educating pregnant women on the role of ITNs in preventing malaria will impact positively in reducing the prevalence of malaria. Furthermore, government should intensify efforts in ensuring that ITNs are readily available at all health facilities and ITNs should be distributed to all pregnant women especially primigravida during ANC visits in order to encourage its utilization.
Placental malaria in this study was associated with increased odds of maternal anaemia. Anaemia remains the most frequent consequence of malaria during pregnancy irrespective of transmission level and pre-pregnancy level of malaria immunity (Menendez, Reference Menendez1995). However, it is difficult to attribute this prevalence of anaemia solely to the effect of malaria parasitaemia as other causes such as helminthiasis, malnutrition, HIV infection and sickle cell anaemia were not assessed in our study.
In our study IPTp-SP use was based on documentation from antenatal cards. That is not likely accurate given that its administration is not often directly observed in the antenatal clinic affecting hence compliance with IPTp-SP. Poor compliance with IPTp-SP may subsequently explain the fact that the use of at least 2 doses of IPTp-SP was not associated with a reduction of the prevalence of placental malaria infection in the study population. However many studies have shown IPTp-SP to be highly effective in reducing placental malaria infection in pregnant women in Burkina Faso (Sirima et al. Reference Sirima, Cotte, Konaté, Moran, Asamoa, Bougouma, Diarra, Ouédraogo, Parise and Newman2006; Gies et al. Reference Gies, Coulibaly, Ouattara, Ky, Brabin and D'Alessandro2008, Reference Gies, Coulibaly, Ouattara and D'Alessandro2009; Tiono et al. Reference Tiono, Ouedraogo, Bougouma, Diarra, Konaté, Nébié and Sirima2009) and in Africa (van Eijk et al. Reference Van Eijk, Ayisi, ter Kuile, Otieno, Misore, Odondi, Rosen, Kager, Steketee and Nahlen2004; Aziken et al. Reference Aziken, Akubuo and Gharoro2011; Vanga-Bosson et al. Reference Vanga-Bosson, Coffie, Kanhon, Sloan, Kouakou, Eholie, Kone, Dabis, Menan and Ekouevi2011). The inconsistent findings on the protective effect of IPTp-SP against placental malaria parasitaemia have been reported in Cameroon (Tonga et al. Reference Tonga, Kimbi, Anchang-Kimbi, Nyabeyeu, Bissemou and Lehman2013), Tanzania (Harrington et al. Reference Harrington, Mutabingwa, Kabyemela, Fried and Duffy2011) and Uganda (Arinaitwe et al. Reference Arinaitwe, Ades, Walakira, Ninsiima, Mugagga, Patil, Schwartz, Kamya, Nasr, Chang, Filler and Dorsey2013). The prevalence of quadruple mutation (combined triple Pfdhfr mutation (51 + 59 + 108) and Pfdhps 437 mutation) in pregnant women was 31·6% in Bobo-Dioulasso (unpublished observations). Therefore, further studies need to be carried out to establish whether the inconsistency of IPTp-SP effect is attributable to loss of SP efficacy in our study area or not.
The prevalence of congenital malaria in our study (0·9%) was very low compared with that previously reported in rural area of Burkina Faso (1·4%) by (Ouédraogo et al. Reference Ouédraogo, Tiono, Diarra, Bougouma, Nébié, Konaté and Sirima2012). The prevalence of congenital malaria assessed by microscopy in sub-Saharan Africa has been reported to vary from 0·4 to 54·2% (Falade et al. Reference Falade, Olugbenga, Henrietta, Adeola, Adegoke, Tagbo, Maman, Davidson and Michael2007; Mwaniki et al. Reference Mwaniki, Talbert, Mturi, Berkley, Kager, Marsh and Newton2010; Enweronu-laryea et al. Reference Enweronu-laryea, Adjei, Mensah, Duah and Quashie2013; Tonga et al. Reference Tonga, Kimbi, Anchang-Kimbi, Nyabeyeu, Bissemou and Lehman2013). The mothers of the three infected newborns are primigravida and secundigravida. It has been shown that primigravida and secundigravida with placental malaria parasitaemia are at increased risk for congenital infection (Malhotra et al. Reference Malhotra, Mungai, Muchiri, Kwiek, Meshnick and King2006; Okafor et al. Reference Okafor, Oguonu and Onah2006). Indeed, the low maternal malarial IgG antibodies transferred to the fetus during pregnancy due to poor immunity to malaria in mothers is a plausible explanation to transplacental transmission of the parasite from mother to child (Taylor and Siddiqui, Reference Taylor and Siddiqui1982). The three mothers had benefited from the doses of IPTp-SP according to the WHO guidelines (WHO, 2004). However, all of them were infected and their babies as well. This observation could be explained by a poor observance of chaemoprophylaxis or a resistance of P. falciparum to SP. The strong correlation between peripheral and placental parasitaemia and the occurrence congenital malaria infection previously reported by (Redd et al. Reference Redd, Wirima, Steketee, Breman and Heymann1996; Okafor et al. Reference Okafor, Oguonu and Onah2006; Ouédraogo et al. Reference Ouédraogo, Tiono, Diarra, Bougouma, Nébié, Konaté and Sirima2012) has been confirmed in this study. Therefore, babies born from mothers with malaria should be screened for congenital malaria.
The overall prevalence of LBW (11·6%) was similar to the prevalence reported from health facilities in Bobo-Dioulasso in 2013 (10·9%) (Ministry of Health of Burkina Faso, 2014). The prevalence of LBW tended to be higher in babies born from mothers with placental malaria parasitaemia compared with that of babies born from uninfected mothers although such difference did not reach statistical significance. The more likely explanation is that our small sample size might have not provided enough strength to our statistics. However previous studies in Africa had found an association between placental malaria parasitaemia and LBW and had defined placental malaria parasitaemia as a predictor of LBW (Menendez et al. Reference Menendez, Ordi, Ismail, Ventura, Aponte, Kahigwa and Font2000; Shulman et al. Reference Shulman, Marshall, Dorman, Bulmer, Cutts, Peshu and Marsh2001; Akanbi et al. Reference Akanbi, Odaibo and Ademowo2009). In this study, primigravida and secundigravida were at higher odds of LBW than multigravida. This is consistent with several reports in Africa (Akum et al. Reference Akum, Anchang, Jacob, Boyo, Mokube and Marita2005; Sirima et al. Reference Sirima, Cotte, Konaté, Moran, Asamoa, Bougouma, Diarra, Ouédraogo, Parise and Newman2006; Gies et al. Reference Gies, Coulibaly, Ouattara and D'Alessandro2009). IPTp-SP was not associated with a reduction in LBW as shown in Ivory Coast (Vanga-Bosson et al. Reference Vanga-Bosson, Coffie, Kanhon, Sloan, Kouakou, Eholie, Kone, Dabis, Menan and Ekouevi2011), in Cameroon (Tonga et al. Reference Tonga, Kimbi, Anchang-Kimbi, Nyabeyeu, Bissemou and Lehman2013) and in Uganda (Arinaitwe et al. Reference Arinaitwe, Ades, Walakira, Ninsiima, Mugagga, Patil, Schwartz, Kamya, Nasr, Chang, Filler and Dorsey2013). However, some studies had shown that the use of IPTp-SP reduced significantly the prevalence of LBW (Sirima et al. Reference Sirima, Cotte, Konaté, Moran, Asamoa, Bougouma, Diarra, Ouédraogo, Parise and Newman2006; Gies et al. Reference Gies, Coulibaly, Ouattara, Ky, Brabin and D'Alessandro2008, Reference Gies, Coulibaly, Ouattara and D'Alessandro2009; Tiono et al. Reference Tiono, Ouedraogo, Bougouma, Diarra, Konaté, Nébié and Sirima2009). The gestational age of women when first dosed of IPTp-SP was given as well as other factors that could explain the occurrence of LBW such as women nutritional status were not collected in this study. This makes the comparison between our study and others difficult.
Although the major strengths of this study were that the study design and the sample size were adequate to estimate the prevalence of placental malaria parasitaemia among the study population we do acknowledge some limitations. First, the selected health facilities may not be representative of all delivery facilities across Burkina Faso. Second, we were unable to obtain accurate data on gestational age limiting hence the assessment of the impact of placental malaria on gestational age.
Concluding remarks
Placental malaria is frequent particularly in primigravida. The results of our study have confirmed that placental malaria parasitaemia is still an important cause of morbidity in pregnancy in Bobo-Dioulasso. We found no evidence for an association between the number of SP doses and the outcome of pregnancy despite a high IPTp-SP coverage. The use of bed nets was linked with a lower level of placental malaria. Delivery of insecticide-treated bed nets should be therefore emphasized in primigravida to reduce the burden of malaria in pregnancy.
ACKNOWLEDGEMENTS
We thank all midwives and pregnant women of both Kua and Lafiabougou health centers.
FINANCIAL SUPPORT
This work was supported by the Université Polytechnique de Bobo-Dioulasso as part of Mamoudou Cisse's PhD project.
CONFLICT OF INTEREST
None.
ETHICAL STANDARDS
The authors assert that all procedures contributing to this work comply with the ethical standards of the Ministry of Health of Burkina Faso.









