The high prevalence of chronic and acute infectious diseases among Egyptian children living in poor areas is known to impair linear growth. Helicobacter pylori, discovered just two decades ago by Warren and MarshallReference Marshall and Warren1 and colonising the mucosal lining of the stomach, is considered one of the most common bacterial infections in manReference Holcombe, Omotata, Eldridge and Jones2, Reference Everhart3, and one such infection that is linked to failure-to-thrive in childrenReference Takahashi, Kimura and Watanabe4.
H. pylori infection causes depressed gastric acid secretion with subsequent loss of the gastric acid barrier and is an aetiological agent of chronic gastritis, gastric and duodenal ulcers, enteric infections and gastric cancerReference Gold, Colletti, Abbott, Czinn, Elitsur and Hassall5, 6. In addition, H. pylori infection has also been reported to have extra-digestive consequences including the retardation of growth rateReference Dale, Thomas, Darboe, Coward, Harding and Weaver7–Reference Bravo, Mera, Reina, Pradilla, Alzate, Fontham and Correa9, the development of iron deficiencyReference Kostaki, Fessatou and Karpathios10 or bothReference Choe, Kim and Hong11. This may result from malnutrition, as infections decrease food intake, impair nutrient absorption, cause direct nutrient losses, increase metabolic requirements and possibly impair the transport of nutrients to target tissues.
Although other reservoirs, such as water, have been proposedReference Klein, Graham, Gaillour, Opekun and Smith12, humans appear to be the primary natural reservoir of H. pylori infection. The route of transmission of H. pylori in man is postulated to be faecal–oral, gastric–oral (in vomitus) or from the consumption of contaminated vegetablesReference Hopkins, Vial, Ferreccio, Ovalle, Prado and Sotomayor13. Residence in a developing country, poor socio-economic conditions, transmission of H. pylori from infected parents to their childrenReference Rothenbacher, Winkler, Gonser, Adler and Brenner14 and overcrowding in free-living and institutionalised childrenReference Vincent, Gottrand, Pernes, Husson, Lecomte-Houcke and Turck15, Reference Galpin, Whitaker and Dubiel16 are among the risk factors for acquiring the infection.
The aims of the present study were to assess the prevalence and risk factors of H. pylori among Egyptian children from different socio-economic classes, and to determine the relationship between growth indicators, such as standing height and weight, and H. pylori infection.
Materials and methods
Design and subjects
A cross-sectional, population-based study was performed among 286 apparently healthy children (mean age 11.04 ± 0.19 years, range 6–15 years) attending public schools in Cairo, Giza or Sohag. Following initial screening, children with a history of H. pylori eradication or antacid use, liver disease or diabetes, with malformations or serious chronic diseases, inflammatory bowel disease or previous gastrointestinal surgery, were excluded. The study protocol was approved by the Committee on the Protection of Human Subjects of the National Research Center and by the School Health Departments. Informed consent was obtained from every parent before children were enrolled into the study.
The schools were divided into four well-defined areas generally classified as affluent suburbs (Heliopolis, Cairo), mixed housings of poor society (Mansheyet Nasser, Cairo), institutions (Giza) and upper rural Egypt (Sohag).
The parents of the children enrolled in the study responded to a structured questionnaire with the help of schoolteachers to obtain parental data on occupation, education level and environmental information, such as the number of persons living in the house, type of house, number of rooms in the house, availability of potable water and sanitary conditions. Education and occupational levels of both parents were recorded and used to define the index of socio-economic class. A score of 1 was given to children whose school was in a deprived area, score 2 for children with more than three persons per room at home, and score 3 for children whose fathers were university graduates and were attending school in a high-class area.
[13C]Urea breath test
H. pylori infection was assessed by use of the [13C]urea breath testReference Rowland, Lambert, Gormally, Daly, Thomas and Hetherington17 following an overnight fast. A baseline breath sample was collected by blowing into a 10 ml Vacutainer. A solution containing 50 mg [13C]urea (99 atom%; Cambridge Isotope Laboratory) was ingestedReference Galpin, Whitaker and Dubiel16. Breath samples were obtained at 30 min thereafter.
13C isotopic enrichment of the breath samples was measured, without further preparation, at MRC-Human Nutrition Research, Cambridge UK, using continuous-flow isotope ratio mass spectrometry (AP2003 IRMS; Analytical Precision Ltd) against a calibrated reference gas, and the results expressed as ‰ relative to PDB (Pee Dee Belemnite), an international standard of known 13C composition. Using cluster analysis of a large dataset of breath test results, 13C enrichment in excess of baseline of exhaled CO2 of more than 5.4 deltas at 30 min after urea ingestion was defined as a positive result for H. pylori infectionReference Mion, Rosner, Rousseau and Minaire18, Reference Thomas, Dale, Harding, Coward, Cole and Sullivan19.
Anthropometric measurements
Information on age was obtained from the birth certificates. Body weight was measured using electronic scales and height was obtained for all children using mobile routine instruments. The standard deviation scores (Z-scores) relative to the National Center for Health Statistics (NCHS) international standardsReference Hamill, Drizd, Johnson, Reed, Roche and Moore20 for weight-for-age (WAZ), height-for-age (HAZ) and body mass index (BMI)-for-age (BMIZ) were calculated using the Epi Info software program, version 10 (Centers for Disease Control and Prevention, 2000). Percentile scores of weight-for-age (WAP), height-for-age (HAP) and BMI-for-age (BMIP) were obtained using Epi Info and standard paediatric height and weight chartsReference Hamill, Drizd, Johnson, Reed, Roche and Moore20.
Statistical analysis
A statistical analysis program (SPSS version 10; SPSS, Inc.) was used to analyse the data. Quantitative data were expressed as means with standard error. Differences between means were evaluated using paired t-tests. One-way analysis of variance was used to compare between groups. The χ 2 test was used in the analysis of contingency tables. The effects of H. pylori infection on growth were analysed by multiple regression analysis using weight, height, WAP, HAP, BMIP, WAZ, HAZ and BMIZ as outcome variables. The number of persons per room (crowding index) and parental social class were analysed as both continuous and categorical variables. Statistical analyses were performed using Fisher’s exact, Student’s t, Mann–Whitney U, Kruskal–Wallis, Spearman rank and Wilcoxon signed ranks tests, as appropriate. A P-value of 0.05 or less was taken to signify statistical significance.
Results
Prevalence of H. pylori infection
The overall prevalence of H. pylori infection in Egyptian schoolchildren was 72.38% (Table 1). There was no significant difference in the prevalence of infection between boys and girls (73.80% vs. 70.34% respectively, odds ratio = 1.05, P = 0.63), or in the independent effect of sex by age (P > 0.1) (Figs 1a and 1b).
Table 1 Effect of infection with Helicobacter pylori on anthropometric measurements among Egyptian schoolchildren

WAZ – weight-for-age Z-score; HAZ – height-for-age Z-score; BMIZ – body mass index Z-score; WAP – weight-for-age percentile score; HAP – weight-for-age percentile score; BMIP – body mass index percentile score.
Values are mean ± standard error of the mean.
* P < 0.05 within groups; NS indicates no significant difference within groups.
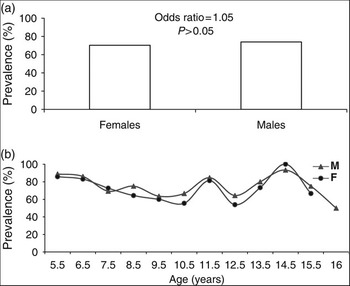
Fig. 1 Prevalence of Helicobacter pylori infection according to (a) sex and (b) age
Geographical location and socio-economic status both had a significant impact on the prevalence of infection. Of schoolchildren living in Sohag, 96.7% tested positive for H. pylori, compared with 81.3% of children from Giza and 61.9% from Cairo (Fig. 2a). Prevalence was also highest among children of low socio-economic class and decreased gradually among children of medium to high socio-economic class (for the whole study population: χ 2 = 73.94, P = 0.00; for children in Cairo: χ 2 = 49.67, P = 0.00). Within Cairo governorate (Fig. 2b), children of low or moderate socio-economic status had a relative risk of 2.56 and 1.98, respectively, for infection compared with those of high socio-economic status (χ 2 = 47.5, P = 0.000; χ 2 = 7.5, P = 0.005).

Fig. 2 Prevalence of Helicobacter pylori infection by (a) site of residence and (b) socio-economic status (χ 2 test for trend: within Cairo – χ 2 = 49.67, P = 0.000; total – χ 2 = 73.94, P = 0.000)
Table 1 shows the mean body weight, height and BMI among schoolchildren with or without H. pylori infection. Both weight and height were significantly lower with H. pylori infection, for boys, girls and the study population as a whole. Figure 3 also illustrates this, with the mean body weight and height for boys and girls distributed according to infection status and age. From this figure, it is evident that the differences in height and weight with H. pylori infection are more noticeable in boys than girls.

Fig. 3 Mean body weight and height by age, sex and Helicobacter pylori infection (M – male; F – female; Free – no H. pylori infection; Inf – H. pylori infection present)
Chi-square tests indicated that the prevalence of stunted growth was significantly higher in the infected children than in children free of H. pylori: 23.67% of infected children (odds ratio = 4.59, 95% confidence interval 1.66–13.68) had HAP below the 5th percentile compared with only 6.33% among the children without infection. Similarly, the proportion with HAZ below –2.0 was significantly higher in the children infected with H. pylori than in the children without infection (Table 1, H. pylori (+) vs. H. pylori (–): 14.98% vs. 2.53%, χ 2 = 8.64, P = 0.003).
WAZ and WAP values also signified reduced growth associated with children with H. pylori compared with children free of the infection (Table 1).
Finally, Fig. 4 shows a shift to the left in the normal distribution of HAZ, WAZ and BMIZ in the infected group compared with the respective distribution in non-infected children or the reference data provided by the NCHSReference Hamill, Drizd, Johnson, Reed, Roche and Moore20 for a healthy population. This again indicates a relationship between H. pylori infection and stunted growth.
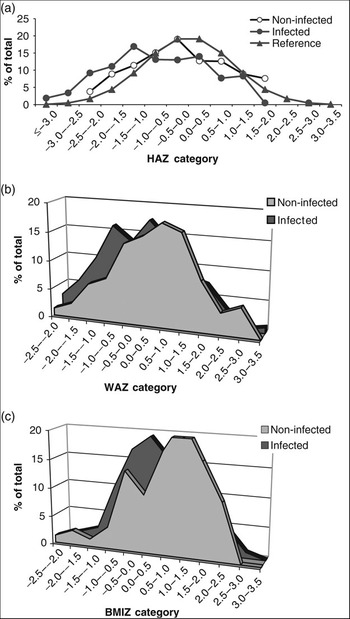
Fig. 4 Distribution of (a) height-for-age Z-score (HAZ), (b) weight-for-age Z-score (WAZ) and (c) body mass index-for-age Z-score (BMIZ) among the study group in relation to infection with Helicobacter pylori
Discussion
H. pylori is one of the most common human bacterial infections in the world, and children in developing countries acquire it early in life. Traditional diagnostic tools such as endoscopy and serology can be too invasive for children but the [13C]urea breath test (UBT) offers a suitable alternative. The UBT is a safe, non-invasive test and has been used successfully for serial determination of the presence of H. pylori prior to and following antibiotic therapyReference Sabbi, De Angelis, Colistro, Dall’Oglio, diAbriola and Castro21. It was the authors’ opinion therefore that the UBT was the most appropriate method for the present study.
Our findings demonstrate that infection with H. pylori is alarmingly high among Egyptian schoolchildren aged 6–15 years, with an overall prevalence of 72%. This ranged from approximately 62% of children in Cairo to 96% of children from rural Sohag, and is much higher than the 46% reported in a recent study of 2–17-year-old Egyptian children using endoscopyReference Sherif, Mohran, Fathy, Rockabrand, Rozmajzl and Frenck22. No gender differences in H. pylori infection were found, although the prevalence did vary with age in both sexes. The underlying cause of the fluctuation in H. pylori incidence with age is unclear from the present study, as results were collated from a single UBT in different children in different age brackets rather than repeated UBTs in the same child at different ages. However, the age-dependent prevalence has been reported elsewhere, where it was hypothesised that children have transient H. pylori infections with high seroreversion ratesReference Naficy, Frenck, Abu-Elyazeed, Kim, Rao and Savarino23–Reference Mitchell, Silva, Barrett, Lima and Guerrant25.
One of the most marked observations from this study was the effect geographical location and socio-economic status had on the relative risk and prevalence of infection among the schoolchildren of Egypt. Previously, the association of overcrowding with H. pylori positivity was consistent with interpersonal spread of infection within the home. This, along with the absence of a fixed hot water supply, had been considered a strong risk factor for infectionReference Webb, Knight, Greaves, Wilson, Newell and Elder26. In contrast, studies which could be seen to reflect good hygienic conditions and less crowded environments reported a much lower prevalence; less than 6 or 7% in a study carried out on 3300 German childrenReference Richter, List, Mueller, Deutscher, Uhlig and Krumbiegel27, with comparable values reported from other European countriesReference Blecker, Lanciers, Hauser and Vandenplas28–Reference Oderda, Palli, Saieva, Chiorboli and Bona30. In more recent times however, acquisition away from the home has been considered more important. Attending school in a deprived area and originating from low parental socio-economic class predispose children to a high risk of infection with H. pylori. In support of this, our data show that features of the community, such as schools in poor areas, rather than those of the home are important in determining H. pylori positivity among children in Cairo.
Whilst H. pylori infection is strongly associated with gastritis, peptic ulcer and gastric cancer in adults, there is also evidence to suggest that in newborns and infants it is linked to diarrhoea, malnutrition, failure-to-thrive and stunted growthReference Thomas, Dale, Bunn, Harding, Coward and Cole31. In our paediatric population, significant differences were found in both weight and height between those with H. pylori infection and those without. Boys and girls testing positive for H. pylori were found to be significantly lighter and shorter than those free of the infection. The lack of weight gain in these children can be explained by the fact that when infected with H. pylori, the amount of gastric acid in a child’s stomach is reduced, weakening the defences against other infections. In developing countries where infection rates are high, recurring problems at an early age lead to diarrhoea, malnutrition and growth impairment. Exposure to microbes, both exogenous pathogens and endogenous biota, has also been reported to be a critical environmental determinant in the expression of heightReference Beard32. Height variations in adulthood, within and between populations, result from a complex interaction between genetic and environmental factors. Experimental studies and historical changes in height, in relation to presumed microbial transmission, support this hypothesisReference Beard32. The lower growth rates seen in this study among H. pylori-infected children, in comparison with growth rates in non-infected age- and sex-matched children, again corroborate results obtained worldwideReference Passaro, Taylor, Gilman, Cabrera and Parsonnet8, Reference Mion, Rosner, Rousseau and Minaire18, Reference Oderda, Palli, Saieva, Chiorboli and Bona30, Reference Beard32–Reference Demir, Saltik, Kojak, Yoce, Quzen and Grakan35. One group in Germany demonstrated statistically significant differences in both height and weight measurements between H. pylori-infected German boys and their H. pylori-negative counterpartsReference Richter, List, Mueller, Deutscher, Uhlig and Krumbiegel27, while a follow-up study carried out in 12–60-month-old Colombian children showed constant deceleration in growth velocity by approximately 0.5 cm per year in infected children compared with those who remained infection-freeReference Bravo, Mera, Reina, Pradilla, Alzate, Fontham and Correa9.
In addition to the detrimental effects H. pylori has on the gastric acid barrier, research groups have been examining the potential link between ghrelin and the infection. Ghrelin, produced in stomach, is a hormone that stimulates food intake and weight gain, but controversy exists over the relationship between ghrelin release and H. pylori infection. Decreased plasma levels of circulating ghrelin have been reported in H. pylori-positive individuals compared with non-infected subjects (194.2 ± 90.2 vs. 250.4 ± 84.1 fmol ml–1 respectively, P < 0.05)Reference Isomoto, Ueno, Nishi, Wen, Nakazato and Kohno36, and following H. pylori eradication, ghrelin levels increase significantlyReference Isomoto, Ueno, Nishi, Wen, Nakazato and Kohno36. Decreased ghrelin concentrations are also associated with higher levels of leptinReference Isomoto, Ueno, Nishi, Wen, Nakazato and Kohno36, a hormone responsible for suppressing appetite, regulating energy intake and modulating energy expenditure, and a reduction in the secretion of growth hormoneReference Cummings and Shannon37. There is some evidence therefore that the negative association between ghrelin and H. pylori infection may be contribute to malnutrition and growth retardation.
To the authors’ knowledge, this is the first systematic study carried out in Egypt to gauge the prevalence of H. pylori infection in a general paediatric population and in which the determinant factors in this important segment of the population are assessed. Such information is of utmost importance to public health authorities before any action regarding this issue can be undertaken. Based on the level of prevalence of H. pylori infection in Egyptian schoolchildren obtained in the present study, and the relationship between this and stunted growth, it is recommended that screening and treatment programmes for H. pylori among asymptomatic children in socially deprived areas be considered as a way forward in tackling this problem.
Acknowledgements
Sources of funding: This study was supported by the Department for International Development (DFI) Higher Education Links scheme through the British Council, Cairo, Egypt (Subject category: Lowering Morbidity Among the Underprivileged Through Clean Environment and Improved Micronutrient Status).
Conflict of interest declaration: The authors declare that they have no involvements that might raise the question of bias in the work reported, or in the conclusions, implications or opinions reported.
Authorship responsibilities: M.A.M. was responsible for conducting all of the fieldwork (anthropometric measurements, administration of [13C]urea to study subjects, collection of breath samples), measurement and analysis of 13C/12C ratio of CO2 in the collected breath samples using the isotope ratio mass spectrometer (IRMS), statistical analysis of the data, and helped in the preparation of the manuscript. L.H. conceived the study, was responsible for the study design, work plan and field work, and helped in statistical analysis of the data and preparing the manuscript. A.C. provided laboratory facilities for measuring the 13C-labelled CO2 using the IRMS. S.J.J. helped in the IRMS measurements of 13C-labelled CO2 and in the preparation of the manuscript.









