Article contents
X-ray powder diffraction analysis of two new magnesium selenate hydrates, MgSeO4·9H2O and MgSeO4·11H2O
Published online by Cambridge University Press: 08 May 2015
Abstract
Several hitherto unknown hydrates of magnesium selenate have been formed by quenching aqueous solutions of MgSeO4 in liquid nitrogen. MgSeO4·11H2O is apparently isostructural with the mineral meridianiite (MgSO4·11H2O), being triclinic, 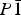 $P{\rm \bar 1}$, Z = 2, with unit-cell parameters a = 6.779 00(8) Å, b = 6.965 16(9) Å, c = 17.4934(2) Å, α = 87.713(1)°, β = 89.222(1)°, γ = 63.121(1)°, and V = 736.15(1) Å3 at −25 °C. MgSeO4·9H2O represents a new hydration state in the MgSeO4–H2O system; it is monoclinic, space-group P21/c, Z = 4, with unit-cell parameters a = 7.270 24(6) Å, b = 10.510 94(9) Å, c = 17.4030(2) Å, β = 109.447(1)°, and V = 1254.02(1) Å3 at −22 °C. The heavy-atom structure of MgSeO4·9H2O has been determined by direct-space methods from X-ray powder diffraction data and consists of isolated Mg(H2O)62+ octahedra and SeO42− tetrahedra linked by hydrogen bonds. The remaining three water molecules occupy the space between the polyhedral ions, contributing to the H-bonded network, which comprises 4-, 5-, and 6-membered rings. A third phase has been observed to crystallise prior to the 11-hydrate upon warming of liquid-nitrogen-quenched glass, but this transforms rapidly to the meridianiite-structured 11-hydrate and the identity of this phase is unclear.
$P{\rm \bar 1}$, Z = 2, with unit-cell parameters a = 6.779 00(8) Å, b = 6.965 16(9) Å, c = 17.4934(2) Å, α = 87.713(1)°, β = 89.222(1)°, γ = 63.121(1)°, and V = 736.15(1) Å3 at −25 °C. MgSeO4·9H2O represents a new hydration state in the MgSeO4–H2O system; it is monoclinic, space-group P21/c, Z = 4, with unit-cell parameters a = 7.270 24(6) Å, b = 10.510 94(9) Å, c = 17.4030(2) Å, β = 109.447(1)°, and V = 1254.02(1) Å3 at −22 °C. The heavy-atom structure of MgSeO4·9H2O has been determined by direct-space methods from X-ray powder diffraction data and consists of isolated Mg(H2O)62+ octahedra and SeO42− tetrahedra linked by hydrogen bonds. The remaining three water molecules occupy the space between the polyhedral ions, contributing to the H-bonded network, which comprises 4-, 5-, and 6-membered rings. A third phase has been observed to crystallise prior to the 11-hydrate upon warming of liquid-nitrogen-quenched glass, but this transforms rapidly to the meridianiite-structured 11-hydrate and the identity of this phase is unclear.
- Type
- Technical Articles
- Information
- Copyright
- Copyright © International Centre for Diffraction Data 2015
References
- 6
- Cited by





