Introduction
Evidence-based conservation management is essential in dynamic environments that require constant re-evaluation in response to changing conditions (e.g. Sutherland et al., Reference Sutherland, Pullin, Dolman and Knight2004; Pullin & Knight, Reference Pullin and Knight2009). Recent studies have shown the utility of dynamic protected areas at local and regional scales in terrestrial and marine environments (e.g. Howell et al., Reference Howell, Kobayashi, Parker, Balazs and Polovina2008; Hooker et al., Reference Hooker, Canadas, Hyrenbach, Corrigan, Polovina and Reeves2011), facilitating both economic needs and environmental protection (Howell et al., Reference Howell, Kobayashi, Parker, Balazs and Polovina2008; Pearce et al., Reference Pearce, Kirk, Lane, Mahr, Walmsley and Casey2008). However, although scientific evidence is available to inform conservation practice (e.g. Pullin & Knight, Reference Pullin and Knight2009; Hooker et al., Reference Hooker, Canadas, Hyrenbach, Corrigan, Polovina and Reeves2011), static forms of conservation practice based on experience, precaution or anecdotal information remain common (e.g. Hyrenbach et al., Reference Hyrenbach, Forney and Dayton2000; Thompson et al., Reference Thompson, Wilson, Grellier and Hammond2000; Schofield et al., Reference Schofield, Hobson, Lilley, Katselidis, Bishop, Brown and Hays2010).
Beaches are of both economic and environmental importance (Arianoutsou, Reference Arianoutsou1988; James, Reference James2000; Schlacher et al., Reference Schlacher, Dugan, Schoeman, Lastra, Jones and Scapini2007), and effective beach management requires regular seasonal and/or annual re-evaluation because beaches are constantly being reshaped by wave action (Kamel & Mrosovsky, Reference Kamel and Mrosovsky2004) and anthropogenic activities (Weishampel et al., Reference Weishampel, Bagley, Ehrhart and Rodenbeck2003). Conservation efforts for loggerhead marine turtles Caretta caretta, categorized as Endangered on the IUCN Red List (Marine Turtle Specialist Group, 1996), tend to focus on nesting beaches, where reproductive output may be assessed and protected (e.g. Hays et al., Reference Hays, Mackay, Adams, Mortimer, Speakman and Boersma1995). Numerous studies have identified the environmental parameters required for successful beach selection, nest site selection and egg development (e.g. Carr & Ogren, Reference Carr and Ogren1960; Hays et al., Reference Hays, Mackay, Adams, Mortimer, Speakman and Boersma1995; Wood & Bjorndal, Reference Wood and Bjorndal2000; Fuentes et al., Reference Fuentes, Limpus and Hamann2010).
Human threats may potentially influence offspring phenotype or cause direct mortality of marine turtles (e.g. Mortimer, Reference Mortimer1990; Pfaller et al., Reference Pfaller, Limpus and Bjorndal2008) through shading (e.g. beach furniture), compaction (e.g. foot or vehicular traffic), or digging/piercing (e.g. umbrellas) of unmarked nests. However, this information has not yet been used to resolve conflicting issues between safeguarding marine turtle nests and recreational demands (James, Reference James2000). However, guidelines and criteria based on scientific research are essential to facilitate the implementation and evaluation of the effectiveness of management options (e.g. Sutherland et al., Reference Sutherland, Pullin, Dolman and Knight2004; Pullin & Knight, Reference Pullin and Knight2009).
Here, we develop evidence-based management recommendations for the nesting beaches of an internationally important loggerhead turtle rookery on Zakynthos, Greece, which is visited by several hundred thousand tourists each summer (May–October), when marine turtle nesting, nest incubation and hatching occur (Arianoutsou, Reference Arianoutsou1988; Margaritoulis, Reference Margaritoulis2005; Schofield et al., Reference Schofield, Bishop, Katselidis, Dimopoulos, Pantis and Hays2009). Both elevation (Wood & Bjorndal, Reference Wood and Bjorndal2000) and beach width (Mazaris et al., Reference Mazaris, Matsinos and Margaritoulis2006) have been suggested as the major regulating factors for nest placement by loggerhead turtles. We investigate which of these two parameters has the greatest influence on emergence and nesting location, and hence the greatest utility for management. We then evaluate the overlap of tourism with nesting parameters, to provide practical, science-based suggestions to reduce anthropogenic pressure. Finally, we discuss the value of evidence-based management as a tool for regulating conservation management of threatened species in dynamic protected areas that are of both environmental and economic importance.
Study area and species
The National Marine Park of Zakynthos lies in Laganas Bay on the Greek island of Zakynthos (Fig. 1). It hosts the largest documented breeding area for loggerhead turtles in the Mediterranean (Margaritoulis, Reference Margaritoulis2005), with a mean of 1,200 nests per year. At this site marine turtle mating activity peaks in mid April (Schofield et al., Reference Schofield, Hobson, Lilley, Katselidis, Bishop, Brown and Hays2010), with females nesting from late May until August. Individual females nest three times per season, after which they return to distant foraging grounds (Zbinden et al., Reference Zbinden, Aebischer, Margaritoulis and Arlettaz2008). The breeding area encompasses six beaches (Marathonisi, Kalamaki, Crystal, Sekania, Daphni and Gerakas), covering a total length of 6.3 km of available nesting habitat. The beaches support varying numbers of nests and differ in environmental conditions and human use (Table 1; Margaritoulis, Reference Margaritoulis2005). Because of the island's location in the Mediterranean, tidal effect is minimal. In general, storms rarely occur after May or before September, when over 80% of nests hatch (Margaritoulis, Reference Margaritoulis2005), so the beaches are not altered by wave action during this period. Visitor access is prohibited on one beach (Sekania) and is regulated on the other five beaches (for protocols see Table 2). Permanent beach furniture is permitted on three of the beaches, with the numbers and location being specified by the Park at the start of each season (Table 2).

Fig. 1 The marine turtle breeding area of Laganas Bay on Zakynthos Island, showing the locations of the six nesting beaches (Marathonisi, Kalamaki, Crystal, Sekania, Daphni and Gerakas). Marine protection zones B and C allow boating at 6 km h−1; boating is prohibited in zone A. The inset shows the location of Zakynthos Island off the south-west coast of mainland Greece.
Table 1 Marine turtle nesting and beach parameters at the six nesting beaches monitored on Zakynthos (Fig. 1) during 2007–2009. The beach width and elevation are measured between the calm sea level and the vegetation line. Means are ± SD.
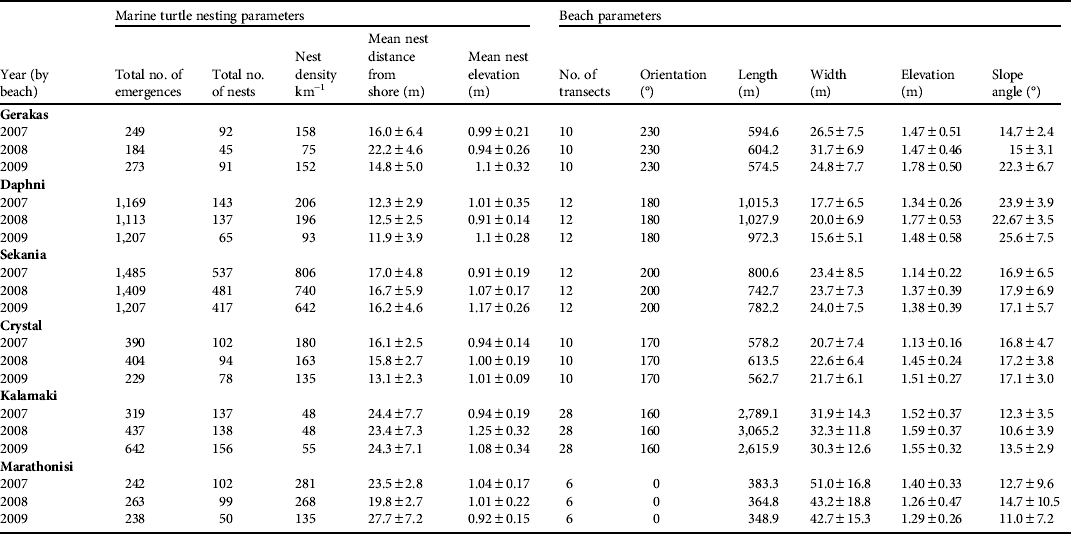
Table 2 Marine turtle and anthropogenic parameters at the six nesting beaches on Zakynthos (Fig. 1). Only data from 1 June to 31 August, between 10.00 and 19.00, were used. Hourly limits were not exceeded in the data recorded in May, September or October. Protected nests indicate caged and/or relocated nests. Numbers in parentheses indicate the number of nests caged.

1 Length of beach covered by beach furniture
2 Range of the distance of beach furniture from the sea
3 Range of the elevation of beach furniture above sea level
4 No data as the number of visitors to this beach is negligible (< 100 visitors over the entire season)
Methods
A global positioning system (GPS) with an accuracy of < 3 m was used to record the position of the following parameters at 2 m intervals: vegetation line (once per year in May), calm sea line (weekly in May–October), wet sand line (i.e. highest tide line, weekly in May–October), storm line (every event where the sea encroached a distance of > 5 m up the beach). This information was transferred to digital maps. In 2007 78 transects traversing the beaches (i.e. from calm sea level to the beach vegetation line, or cliff face or wall) were delineated (using a GPS) at 50 m intervals along all nesting beaches except Kalamaki, on which transects were delineated at 100 m intervals because of the relatively greater length (3 km) of this beach. In 2008–2009 the same transect lines were reassessed for inter-season comparisons. Beach elevation was measured at 3 m intervals along each transect, using a digital theodolite, in May of all 3 years, and repeated in August 2007 to determine beach stability during the summer, with a mean difference across measurements of 0.034 ± SD 0.012 m, indicating that the beaches were stable.
Laying and hatching were monitored during May–October of 2007, 2008 and 2009 through nightly patrols and daily dawn beach surveys. The position of all emergences from the sea and resultant nests were recorded, using a GPS, in relation to markers at 20–50 m intervals along the back of the beach. In all 3 years the minimum number of nests was confirmed by at least one of the following methods: (1) locating eggs for relocation and/or caging the morning after egg laying, (2) matching laid and hatched nest locations, and (3) hatched nests not recorded at egg laying. For nests relocated from their natural locations, the original and new locations were recorded, with the original being used for the purposes of this study. For each nest the distance to the sea was measured at egg laying and hatching. We used a theodolite to measure the elevation of 70% of nests during August of each year. We also measured the elevation above sea level of a sample of 20 caged nests at egg laying and hatching, to confirm that measurements were similar between June and August (mean difference 0.004 ± SD 0.001 m). The spatial distribution of all nesting (turtle tracks that resulted in nests) and non-nesting (turtle tracks that did not result in nests) emergences from the sea onto the shore of the six nesting beaches was evaluated for each 100 m section, using methodology adapted from Weishampel et al., (Reference Weishampel, Bagley, Ehrhart and Rodenbeck2003). We used Moran's I (Griffith, Reference Griffith1988) to determine if there was a significant correlation of the observed nesting and non-nesting emergences at one location with emergences at other sampling locations.
The locations of all anthropogenic features were recorded with a GPS. The area designated for permanent beach umbrellas was mapped in May and rechecked weekly. In addition, the distance from the sea and elevation of the lowest and highest items of furniture were measured. The number of nests caged and left in situ or relocated out of these areas was recorded in each season. The area of beach used by visitors at the busiest hour and day of the week was recorded (weekends during 14.00–16.00; calculated from 2007–2009 National Marine Park of Zakynthos guard data sets). The location of the highest point of the beach used by visitors was recorded, with a GPS, at 2 m intervals on all beaches (except Sekania).
The overlap in the distribution of nests with the area used by people was determined by measuring the number of nests within areas of recorded tourist use by overlaying the GPS data for the two data sets using ArcMap v. 9.1 (ERSI, Redlands, USA). We used beach slope data to calculate the probability of nests occurring in designated beach furniture zones.
Descriptive statistics, linear regression and the coefficient of variation were used to evaluate statistical trends within and between the data sets. Statistical significance was at P < 0.05.
Results
Environmental parameters
The beaches of Sekania, Daphni and Marathonisi had the most stable elevation across the 3 years of study (coefficient of variation, CV, of 0.8, 0.8 and 0.9 in 2007, 2008 and 2009, respectively), followed by Crystal, Gerakas and Kalamaki (CV 0.13, 0.16 and 0.17, respectively). Beach slope varied by a mean of 3.26 ± SD 3.19° between seasons. Slope and distance to sea were negatively correlated (F 1,204 = 199, r 2 = 0.7, P < 0.0001); i.e. beaches with narrower widths had a steeper incline and vice versa. Daphni had the narrowest width and steepest incline and Kalamaki had the greatest width and shallowest incline (Fig. 2a,b; Table 1).

Fig. 2 Variation in (a) beach width, (b) beach slope, (c) number of marine turtle emergences, and (d) number of marine turtle nests on the 78 transects along the total 6.3 km of the six loggerhead turtle nesting beaches in 2007 (dark grey line), 2008 (light grey line) and 2009 (black line). The x-axis represents the total 6.3 km length of nesting beach.
Although beach width varied across the 78 transects (mean 27.4 ± SD 12.3 m, range 7–68 m, CV 0.45; Fig, 2a, Table 1) it remained relatively stable within each beach (CV 0.03–0.09) across years (CV 0.07; Fig. 2a, Table 1). The elevation of the transects was more uniform (mean: 1.45 ± SD 0.4 m, CV 0.28, range 0.73–2.88 m) across all beaches. However, the angle of the slope was variable (mean 16.2 ± SD 6.5, range 3–44°; Fig. 2b, Table 1), being generally consistent across the beaches in all years (CV 0.07) but with a noticeable interannual variation for individual beaches.
Nesting parameters
In total 3,854, 4,015 and 3,154 turtle emergences from the sea were recorded on the six beaches in 2007, 2008 and 2009, respectively, of which 1,113, 994 and 858, respectively, were nests. On average 91.6% of nests were below the vegetation line (2007: 92.7%; 2008: 92.4%; 2009: 89.3%), and the remainder were above the vegetation line. In all 3 years the majority of turtle emergences occurred at Sekania and Daphni; however, although Sekania had the majority of nests in all 3 years, Daphni did not follow a similar trend. Hence, sites selected for emergence may not necessarily be viable for nesting. Overall, Sekania and Marathonisi supported the largest nesting densities (number of nests per km of nesting beach). In addition, these two beaches had the most stable spatial distribution of emergences across years (i.e. emergences occurred at similar locations in different seasons; CV 0.03 and 0.07, respectively) followed by Gerakas, Kalamaki, Crystal and Daphni (CV 0.09, 0.1, 0.2 and 0.33, respectively; Table 1). Sekania and Daphni had the lowest variability in the spatial distribution of nests (CV 0.12 and 0.20, respectively), followed by Gerakas, Kalamaki, Crystal and Marathonisi (CV 0.36, 0.54, 0.62 and 0.74, respectively). The distribution of emergences was strongly correlated with beach slope angle (F1,200 = 121, r2 = 0.63, P < 0.0001), although the correlation between the spatial distribution of nests and beach slope angle was weak (F1,200 = 9, r2 = 0.21, P = 0.002). There were weak negative correlations for beach width and the spatial distribution of emergences (F1,200 = 48, r2 = 0.40, P < 0.001) and the spatial distribution of nests (F1,200 = 15; r2 = 0.27; P = 0.001).
The spatial distribution of all turtle emergences (both nesting and non-nesting) and the resultant nests across years were relatively consistent along the total 6.3 km length of nesting beach (CV 0.04 and 0.1, respectively; Figs. 2c,d, 3a); however, there was noticeable interannual variation for individual beaches (CV 0.03–0.33 and 0.12–0.73, respectively). Track density and the number of resultant nests per 100 m section were correlated (Fig. 3a, trendline 1: F 1,200 = 105, r 2 = 0.6, P < 0.0001). This correlation strengthened when excluding Daphni (Fig. 3a, trendline 2: F 1,170 = 172, r 2 = 0.72, P < 0.0001) or Sekania and Daphni (Fig. 3a, trendline 3: F 1,146 = 493, r 2 = 0.88, P < 0.0001), indicating that parameters other than emergence site may influence nest placement.

Fig. 3 (a) Correlation between resultant nests (as % of total tracks per season) and track density (per 100 m). Line 1 is the correlation for all beaches (F 1,200 = 105, r 2 = 0.6, P < 0.0001); line 2 is for all beaches excluding Daphni (grey circles; F 1,170 = 172, r 2 = 0.72, P < 0.0001); line 3 is for all beaches excluding Sekania and Daphni (white and grey circles respectively; F 1,146 = 493, r 2 = 0.88, P < 0.0001). (b) Nest placement parameters (distance and elevation) with respect to the slope angle of all transects (n = 78) across all six nesting beaches in 2007–2009. Distance to sea (circles) was negatively correlated with increasing beach slope angle (r 2 = 0.47, P < 0.0001), whereas elevation above sea level (triangles) was consistent for all slope types (r 2 = 0.003, P < 0.0001).
Nesting and environmental parameters
Overall, mean nest elevation was 1.08 ± SD 0.4 m (CV 0.37, range 0.3–2.6) and mean nest distance from the sea was 17 ± SD 6.99 m (CV 0.41, range 2–52.6). There was no temporal variation in nest elevation across the nesting period (F 1,1471 = 0.47, r 2 = 0.0001, P = 0.49). Nest elevation and distance to sea were positively correlated for a sample of nests (n = 1,472 nests across all seasons; F 1,1471 = 507, r 2 = 0.26, P < 0.0001). We used the variability in beach slope along transects to evaluate whether turtles nest at the same elevation but travel further from the sea or nest at the same distance from the sea but at a different elevation. We found that with increasing beach slope there was a decline in the distance travelled to the nest (F 1,182 = 121, r 2 = 0.47, P < 0.0001; Fig. 3b). In contrast, nest elevation remained consistent at just over 1 m above sea level across the entire range of beach slope angles (i.e. 0–35°, F 1,182 = 0.46, r 2 = 0.003, P < 0.0001; Fig. 3b). Hence, beach slope is the more reliable indicator of where nests are likely to be placed on a beach in relation to sea level. Using the mean ± SD nest elevation data, the optimal width of the beach area available for nesting was 3.5, 5.5, 7.5, 8.5, 10 and 14.5 m on Crystal, Kalamaki, Daphni, Sekania, Gerakas and Marathonisi, respectively.
During the nesting season (May–September) wave action occurred on the beaches when wind speeds exceeded 22 km h−1, which was determined by seven major beach-washing events, recorded using GPS units, between 2007 and 2009. The mean elevation of the calm sea wet sand line (i.e. high tide) was 0.32 ± SD 0.17 m above sea level, whereas that of summer storms (wind speeds > 22 km h−1) was 0.55 ± SD 0.065 m above sea level. We found that 3–8% of nests fell within the mean summer storm line, with > 80% nests having hatched before September storms in all years. The worst case scenario would be a summer storm > 22 km h−1 from late July to early August (i.e. during the peak period of incubation for this site), whereby all nests below 0.79 m elevation (i.e. maximum recorded storm line) would be washed (but still hatch) or inundated, which would account for 26.7% of all nests in a given season.
Nesting and anthropogenic parameters
In total > 300,000 visitors frequented the nesting beaches open to the public (i.e. excluding Sekania) during June–August of each year. On average, there were 35–227 visitors per hour (Table 2), swelling to 170–985 visitors per hour at peak times (14.00–16.00, Saturday–Sunday, August).
Although only 7% of nests overlapped with beach visitor use each year (i.e. 79, 67 and 72 nests in 2007, 2008 and 2009, respectively; Fig. 4a–f), there was the potential for this figure to reach 36% (Fig. 5a). This is because legal beach furniture zones (Table 2) are up to 20 m from the sea, and elevation above sea level is up to 1.3 m. Of the nests that overlapped with visitor area use 76–81% nests were caged and hence received additional protection (Table 2).

Fig. 4 Example of interseasonal variation in nest distribution versus visitor area use at the nesting beaches of Crystal (a–c) and Marathonisi (d–f) in 2007, 2008 and 2009.

Fig. 5 (a) Percentage of nests (black bars; n = 1,472) and visitor area use (grey bars; n = 2,232) in relation to beach elevation across the 3-year survey period. (b) The percentage of nests that could potentially be afforded protection (black line) and the percentage of beach left open to the general public (grey line) with respect to beach slope parameters.
Because nests are more likely to be placed closer to shore on steeper slopes, the risk of overlap with and damage by tourism is increased. Therefore, we evaluated whether the existing overlap could be further reduced by focusing annual conservation effort (i.e. further reducing human use) on beach sections with the steepest inclines (Fig. 5b). For example, if access to all sections of beach (excluding Sekania) with slopes > 22° was restricted (i.e. by limiting visitor use to passage along the sea line only), 50% of nests would be completely protected at the cost of 1,100 m total beach length (i.e. 20%) currently open to the general public. However, the steepest sections change annually across the nesting area. For example, in 2007–2008 just one 100 m section at Gerakas had a slope > 20°, whereas in 2009 there were six viable sections. The closure of 600 m of this 1,000 m beach would not be practical because of its high visitor load (Table 2). Hence, following identification of the steepest slopes each year, the management agency needs to develop additional criteria to select core sections for additional protection by taking into account actual beach length and visitor use (and hence impact on both nests and tourists).
Discussion
Our results show that the nesting beaches of Zakynthos exhibit a broad spatial range in slope and width characteristics. Annual variation in beach slope characteristics probably results from exposure to strong wind and wave action (e.g. Kamel & Mrosovsky, Reference Kamel and Mrosovsky2004; Fuentes et al., Reference Fuentes, Limpus and Hamann2010) during the non-nesting period (October–April), causing the accretion and erosion of sand and consequently leading to changes in the distribution of emergences of turtles from the sea and resultant nests. With respect to nesting beach selection, our results support that of Mazaris et al., (Reference Mazaris, Matsinos and Margaritoulis2006), who found a weak correlation between nest distribution and beach slope. However, we recorded a strong correlation in the distribution of emergence locations with steep beach slopes (and in general narrower beach widths). We suggest that the difference between distributions of emergences from the sea and resultant nest distributions is because additional parameters influence the outcome of nesting (Godley et al., Reference Godley, Broderick and Hays2001), including the presence of stones, clay and marine debris, and sand characteristics (e.g. Mortimer, Reference Mortimer1990; Weishampel et al., Reference Weishampel, Bagley, Ehrhart and Rodenbeck2003). With respect to nest-site selection, we recorded mean nest elevations of 1 m above sea level, strongly corroborating previous studies (Johannes & Rimmer, Reference Johannes and Rimmer1984; Horrocks & Scott, Reference Horrocks and Scott1991; Wood & Bjorndal, Reference Wood and Bjorndal2000; Weishampel et al., Reference Weishampel, Bagley, Ehrhart and Rodenbeck2003). Hence, beach slope provides a reliable way to identify potential emergence and nest-site positioning for annual adjustment of the management of anthropogenic activities (Hansen et al., Reference Hansen, Hoffman, Drews and Mielbrecht2009).
Our study shows that turtles must respond to annual changes in beach slope characteristics to locate suitable environmental conditions for nesting (e.g. Kolbe & Janzen, Reference Kolbe and Janzen2002; Tucker, Reference Tucker2010). In theory, repeated use of the same habitat may improve fitness as it reduces the cost of search effort (e.g. Encalada et al., Reference Encalada, Bjorndal, Bolten, Zurita, Schroeder and Possardt1998). Hence, fidelity is predicted to arise in areas of high stability and associated high nesting density (Switzer, Reference Switzer1993). For example, the high nesting densities recorded in our study at Sekania may be explained by high fidelity of the adult population because of the stable characteristics of the beach, possibly combined with the effect of natal homing of offspring (Encalada et al., Reference Encalada, Bjorndal, Bolten, Zurita, Schroeder and Possardt1998; Weishampel et al., Reference Weishampel, Bagley, Ehrhart and Rodenbeck2003). In comparison, the large number of failed nesting emergences recorded on the neighbouring beach of Daphni indicates that turtles are not necessarily selecting the most suitable sites to emerge. Hence, fidelity to beach or nest sites may be broad (rather than precise), corroborated by the 70% fidelity to beaches previously recorded for this population (Katselidis et al., Reference Katselidis, Schofield, Margaritoulis, Coyne and Clark2004). However, this strategy requires the expenditure of larger amounts of energy in exploratory emergences, which may reduce the total number of possible nests (and hence fitness) by an individual in a given season. In general, as habitat quality affects individual and offspring fitness, prospecting and using more than one habitat may present a more evolutionary stable strategy (e.g. Switzer, Reference Switzer1993; Kolbe & Janzen, Reference Kolbe and Janzen2002; Tucker, Reference Tucker2010), particularly when information on offspring success rates is not available to the parent, as is the case for marine turtles.
We found that 90% of nests occurred below the vegetation line and 90% of nests occurred above the mean summer storm inundation line of waves. In many species that nest adjacent to water bodies there is a risk of egg loss from inundation by seasonally rising water levels or land erosion by storms (e.g. shorebirds, Frederic, Reference Frederic1987; marine turtles, Pfaller et al., Reference Pfaller, Limpus and Bjorndal2008), penetration by roots (e.g. marine turtles, Wood & Bjorndal, Reference Wood and Bjorndal2000; crocodiles, Leslie & Spotila, Reference Leslie and Spotila2001), and excessive moisture, salinity, desiccation, or increased risk of predation of eggs or offspring (e.g. marine turtles, Wood & Bjorndal, Reference Wood and Bjorndal2000; terrapins, Spencer & Thompson, Reference Spencer and Thompson2003). Trade-offs often occur; for example, when predation pressure is strong individuals may locate nests closer to shore in less suitable habitats (e.g. Spencer & Thompson, Reference Spencer and Thompson2003). Alternatively, some species preferentially select sites with a risk of predation because of the high suitability of the habitat (e.g. Wootton, Reference Wootton1992). On Zakynthos predation risk is minimal (Margaritoulis, Reference Margaritoulis2005) and we found that < 10% of nests are at risk of inundation from summer storms, with only nests laid late in the season (i.e. after mid July) being at risk of inundation from September storms, corroborating the findings of Pike (Reference Pike2007). Based on our evaluation the predicted worst case scenario inundation level was 26.7% of nests, which closely matched the 32% recorded by Margaritoulis (Reference Margaritoulis2005) for the 2002 season during which strong southerly winds occurred from mid July onwards. However, such conditions have only been recorded twice in a 27-year period (Margaritoulis, Reference Margaritoulis2005), and nests of other breeding populations are subject to as much as a 60% risk of inundation (Eckert, Reference Eckert1987; Pike, Reference Pike2007). On Zakynthos mean summer storm inundation risk and vegetation may regulate nest placement to 1 m elevation above sea level, along with other drivers such as moisture levels, salinity and pH (Godley et al., Reference Godley, Broderick and Hays2001; Weishampel et al., Reference Weishampel, Bagley, Ehrhart and Rodenbeck2003), or even the highest spring tide line (Kamel & Mrosovsky, Reference Kamel and Mrosovsky2004). In comparison, the positioning of nests by marine turtle populations elsewhere may be driven by different co-varying forces (Storch & Frynta, Reference Storch and Frynta1999; Pfaller et al., Reference Pfaller, Limpus and Bjorndal2008), resulting in the marked variation recorded in research on nest-site selection (e.g. Eckert, Reference Eckert1987; Hays et al., Reference Hays, Mackay, Adams, Mortimer, Speakman and Boersma1995; Weishampel et al., Reference Weishampel, Bagley, Ehrhart and Rodenbeck2003).
Our study shows that effective beach protection requires information about the locations of all emergences and nests, as both aspects represent different habitat selection criteria at different phases of the nesting process. Because of the changing distribution of emergence and nest locations in response to changing environmental conditions each year the full extent of all nesting beaches should be viewed as potentially viable nesting habitat, with the sections receiving the most protection being adjusted each year (Grantham et al., Reference Grantham, Bode, McDonald-Madden, Game, Knight and Possingham2010). For instance, the delineation of static zones based on nesting and/or emergence data from previous years may not reflect the conditions of a particular year. Beach slope characteristics could be used as a practical and effective tool to predict the key zones used by turtles each year, and thus adjustment of the permitted area used for tourism (visitors and business operators alike; Grantham et al., Reference Grantham, Bode, McDonald-Madden, Game, Knight and Possingham2010; Wintle et al., Reference Wintle, Runge and Bekessy2010).
Beach use is two dimensional, with both beach width, (i.e. sea to vegetation line) and beach length requiring consideration. Under existing management protocols visitors use the entire beach length but use of the width is restricted. Although the overlap between visitors and nests was only 7%, this could potentially reach 40% because of legal beach furniture covering a greater width of the beach. Beach use by people should therefore be limited to areas of lower elevation. On steeper sections elevation is higher closer to the sea, further increasing the risk of tourist use overlapping with nests. This problem could be resolved by closing steeper sections of beach, only allowing pedestrian visitors to pass these sections along the shoreline, similar to beach management for ground nesting birds such as oyster catchers and dotterels (James, Reference James2000). Hence, the characteristics of beach slope in a given year could be used to prioritize the most important sites for protection before the onset of the nesting and tourist season (i.e. in May). However, alternative locations for use by displaced tourists should also be provided, such as allowing larger vertical area use in beach sections with shallower beach slopes. In addition, the impact of beach furniture on nests requires quantification.
Annual measurements of beach slope could also contribute towards (1) calculating changes in nest inundation risk, and hence relocation protocols (Kamel & Mrosovsky, Reference Kamel and Mrosovsky2004; Pike, Reference Pike2007; Pfaller et al., Reference Pfaller, Limpus and Bjorndal2008), (2) interpreting the impact of different wind strengths and directions on the morphology of nesting beaches, to forecast future beach structures and/or identify alternative suitable habitats (Hulme, Reference Hulme2005; Fuentes et al., Reference Fuentes, Limpus and Hamann2010), and (3) modelling how this population may adapt to altered environments as a result of climate change or changing anthropogenic pressure (Weishampel et al., Reference Weishampel, Bagley, Ehrhart and Rodenbeck2003; Hansen et al., Reference Hansen, Hoffman, Drews and Mielbrecht2009; Mazaris et al., Reference Mazaris, Matsinos and Pantis2009). Climate change is likely to lead to more hatchlings because of temperature-dependent sex determination (Hays et al., Reference Hays, Fossette, Katselidis, Schofield and Gravenor2010); hence future work should incorporate nest quality (i.e. hatchling success and/or sex ratio) when assessing how to minimize overlap of turtle nests with beach furniture.
Overall, our study shows that loggerhead turtles have the capacity to respond to environmental variables and nest in habitats that suit their requirements; i.e. preferably on beaches with steep inclines and at an elevation of 1 m above sea level. In turn, the management of such annually dynamic systems must exhibit flexibility. Hence, by identifying the key environmental parameters that drive nest site selection annual changes in these parameters could be monitored and management protocols for the beaches adjusted. In conclusion, this study shows the value of evidence-based management as a practical scientific tool to conserve threatened species in dynamic protected areas that are of both environmental and economic importance.
Acknowledgements
We thank the National Marine Park of Zakynthos Management Agency for permission to conduct this study, and we are grateful for the assistance of National Marine Park of Zakynthos research personnel, National Marine Park of Zakynthos guards and Archelon volunteers. We thank Rick Heron for advice on methodological design and equipment, and Graeme C. Hays and two anonymous reviewers for constructive suggestions.
Biographical sketches
Kostas Katselidis is a manager and researcher at the National Marine Park of Zakynthos, specializing in developing sustainable initiatives within the marine, coastal and terrestrial protected area. Gail Schofield specializes in marine vertebrate tracking, ecology and conservation, primarily of marine turtle breeding and foraging habitats. Giorgos Stamou specializes in population dynamics, life history strategies, social and political ecology, the development of social research methods, and environmental education. Panayotis Dimopoulos specializes in ecology, conservation and management of habitats and species in protected areas, eco-geography and conservation status assessments. John Pantis specializes in the management and conservation of protected areas, the structure and dynamics of ecosystems, landscape and acoustic ecology, and indices of environmental stress.











