Abstract
Purpose
Plasma neurofilament light (NFL) is a marker of neuronal injury, associated with poor neurological outcomes in adults and neonates in a wide range of diseases. We sought to describe the associations between NFL at admission (NFLadm) and outcomes in a heterogenous cohort of critically ill children needing unplanned admission to the pediatric intensive care unit (PICU).
Materials and methods
We analysed prospectively collected biobank samples from critically-ill children with unplanned admissions to PICU. Samples were selected for survivors who had a 12-month follow-up with parent-completed Pediatric Quality of Life Inventory (PedsQL) scores as the primary outcome of interest.
Results
Data from 52 children were analysed. The median NFL was 17.0 pg/ml (IQR 5.8–28.1). There were no significant associations between NFLadm levels and PedsQL (NFLadm-adjusted odds ratio 0.99, 95% CI 0.97, 1.02) at 12 months. NFL showed associations with outcomes at PICU discharge, such as change in functional status and need for organ support but interpretation is limited by sample size.
Conclusions
Admission NFL values were not associated with 12-month quality of life outcomes in a heterogenous cohort of children admitted to PICU.
Similar content being viewed by others
Introduction
Health-related quality of life (HRQL) following paediatric intensive care unit (PICU) admission is an outcome of increasing importance, with the increasing survival following PICU admission [1,2,3,4,5]. HRQL does not return to baseline even after a year in children following admission to PICU with sepsis. Those with abnormal neurological examination or suspected neurological injury during the PICU course are at higher risk of worse HRQL measures. This suggests that neurological injury in PICU may have a longer-term impact of quality of life [6]. This is consistent with reports of poorer HRQL in adults following both traumatic and hypoxic-ischaemic brain injury, with neurocognitive and psychosocial factors recognised as determinants of this in the latter [7, 8].
The lag time between PICU admission and assessment of neurological morbidity makes the association with treatment strategies in PICU difficult to test. Co-morbidities, ongoing disease progression and repeated PICU admissions may also contribute to neurological morbidity. To understand the impact of acute interventions, a time-responsive biomarker, with good prognostic validity for long-term morbidity may be helpful.
Neurofilament proteins are exclusively expressed in neurons as scaffolding proteins. They are released into cerebrospinal fluid following neuroaxonal injury, with a leak into blood [9]. Neurofilament light (NFL) has shown promise as a biomarker of neuronal damage in neurodegenerative disease. Recent studies have demonstrated the predictive validity of NFL for poor outcomes 6 months post-cardiac arrest in adults [10, 11] and neurodevelopmental outcomes of premature infants at 2 years [12,13,14].
In children, NFL has been associated with adverse outcomes (death and severe disability) 12 months post-cardiac arrest [15, 16]. NFL values are reportedly higher in children with milder neurological diseases such as post-status epilepticus [17]. Compared to healthy controls, NFL values are higher in those with neuro-developmental disorders such as autistic spectrum behaviour: in these children, NFL values are associated with symptom severity [18]. Therefore, NFL may be sensitive to a degree of neurological injury or damage not typically identified on more conventional diagnostic tools such as CT or MRI. In addition, recent evidence suggests that NFL may be more sensitive to neurological damage and changes in outcomes compared to more established markers of neurological injury such as S100B and neuron-specific enolase (NSE) [19, 20].
In this study, we hypothesised that neurological injury associated with disease and treatment factors immediately prior to, and within PICU, are associated with worse long-term HRQL measures in children admitted to PICU. To test this, we measured NFL in biobank samples from children who survived an emergency admission to PICU and had follow-up at 12 months post-admission. The primary aim of the study was to explore the association between NFL and outcomes in a heterogenous PICU population. In addition, in a sub-group with a second NFL measurement 24–48 h post-enrolment, we described the change in NFL distribution and explored its association with outcomes. A secondary aim was to describe the distribution of NFL in this heterogenous PICU population, including in the sub-groups with pre-existing morbidity and known or suspected neurological injury.
Materials and methods
Study samples
Blood samples from the multicentre Biomarkers of Acute Serious Illness in Children (BASIC) biobank were used to measure NFL. The methods and cohort profiles of children enrolled in BASIC have been previously described [21]. Briefly, children 0–16 years of age (excluding those < 36 weeks of gestation) were enrolled into BASIC if they (i) were being transported to one of three participating PICUs by the regional intensive care transport service as an emergency admission and (ii) had an indwelling arterial or venous catheter for sampling. Blood and urine samples were collected during stabilisation and transport. Parents were approached for consent to continue in the study within 24–48 h of enrolment (‘deferred consent’). The consent process included options to not consent for the use of any collected samples or data, the use of already collected samples/data but not for any further collection, and full consent to collect further samples and patient data. A second sample was taken after consent was obtained (i.e., 24–48 h following enrolment) if an indwelling blood sampling line was still in situ. Parents were separately asked for consent to be approached 12 months from admission for follow-up [22].
Plasma samples from the biobank were used for NFL measurement from survivors who had follow-ups at 12 months. The admission sample (NFLadm) was used for the main analysis, as more children were likely to have samples from admission than a second sample (owing to death or recovery, removal of sampling lines, refused consent for further sampling). For those who had a second sample (NFLtp2), the difference between the two NFL samples was calculated and associations with outcomes were explored. A sample size was not pre-defined—all available samples were used.
NFL measurements
Blood samples were collected in a sodium citrate tube, centrifuged at 2500 × g for 15 min at 4℃ and stored at − 80℃ as part of the BASIC biobank. NFL was measured using Simoa technology on an HD-X Analyzer, according to the manufacturer’s instructions (Quanterix, Billerica, MA) [23]. Duplicate assays were measured, samples with an intra-assay coefficient of variation > 20%, or pairs with an inter-assay variation coefficient of variation > 20% were excluded.
Twelve-month follow-up post-PICU admission
Follow-up procedures at 12 months post-admission for children recruited to BASIC have previously been described [22]. Follow-up comprised of a general screening questionnaire (whether the parent had any concerns or not), how the child was feeling compared to before the PICU admission (options of better, worse or the same) and two validated questionnaires—the Paediatric Quality of Life Inventory (PedsQL) [24, 25] and the Child Behaviour Checklist (CBCL) [26]. The PedsQL score, as a validated age-specific assessment of the quality of life of the child, was considered most likely to reflect the impact of neuronal loss during critical illness. This was chosen as the primary outcome. As NFL is a marker of axonal damage, and has been associated with white matter changes on MRI in neonates [14], we separately analysed the association of NFL with the total physical health summary score component of PedsQL, which comprised 8 items to measure physical function.
NFL was measured from the banked samples after follow-up data collection. Those analysing the NFL samples were blinded to the follow-up data.
Cohort data collection
Baseline data at recruitment, during transport, through the PICU admission, and outcome data were collected for each child in the BASIC study using a standardised case-report form. A detailed description of the data collection has been described [21]. For this study, demographic data including age, sex, weight, co-morbidities (defined as conditions needing hospital treatment or follow-up) and reason for admission (collected at admission and discharge) were extracted. Physiological markers collected in the first hour of PICU admission as part of the Paediatric Index of Mortality-3 score were used (systolic blood pressure (SBP), partial pressure of arterial oxygen, fraction of inspired oxygen and base excess), in addition to whether the children had a cardiac arrest prior to PICU admission. PICU outcome data included length of stay, organ support duration and type (mechanical ventilation, continuous infusions of vaso-active drugs, continuous renal replacement therapy, or extra-corporeal membrane oxygenation), and neurological imaging or neurophysiological investigations on PICU. The investigation results for all children in the biobank were reviewed by one of the authors (YF) and categorised as normal or abnormal based on clinical context; a second reviewer (PR) categorised cases where there was uncertainty. Functional status at PICU admission (i.e. to represent the pre-morbid state) and discharge was also collected using the Paediatric Overall (POPC) and Paediatric Cerebral Performance Categories (PCPC). Worsening POPC/PCPC between admission and discharge was used as a short-term outcome measure.
The total PedsQL score at 12 months was categorised as low (less than 1 standard deviation below the age-specific mean) or not low [27]. The same was used for the physical health summary score (using the proxy cut-off score of 63.28 [27]). The responses for the other components of the 12-month follow-up questionnaire were categorised as ‘case’ or ‘not case’ (CBCL score ≥ or < 93rd centile) for CBCL; ‘no concerns’ or ‘any concerns’ for the general screening question, and ‘same,’ ‘better’ or ‘worse’ for answers regarding how the child was feeling compared to before the PICU admission.
Data analysis
Baseline characteristics and outcomes of the cohort were described using summary statistics. Categorical variables were described by frequencies and percentages. Continuous variables were described by median and interquartile range, or mean and standard deviation depending on normality. Normality was assessed using the Shapiro-Wilks test. Systolic blood pressure was standardised for age using the LMS method [28] based on normal data from the US Task Force Fourth Report, assuming the 50th centile of height and using centile values for 1-year-olds for children less than 1 year [29]. Missing data were not imputed.
The primary outcome measure was total PedsQL scores at the 12-month follow-up. Secondary outcome measures included the three other measures from the 12-month follow-up; short-term outcomes at PICU discharge: duration of ventilation, organ support free days at 30 days, abnormal neuro-imaging or neurophysiology during PICU admission, length of PICU stay, worsening of POPC and PCPC between admission and discharge.
Univariable associations were tested using non-parametric tests (Mann–Whitney or Kruskal–Wallis) for categorical variables and Spearman’s or Pearson’s correlation coefficient for continuous variables. Bonferroni correction was used for multiple comparisons. The predictive ability of NFL was measured using the area under the receiver operator characteristic curve (AUC ROC). To test how NFL performed against the currently used prognostic indicators of abnormal neuro-imaging/neurophysiology tests, we used DeLong’s method to compare the AUC ROC for each. Long-term outcomes are likely to be confounded by co-morbidities and the course during the PICU. To account for these, logistic regression was used with co-morbidities (as a binary variable) and the number of organ support free days in PICU at 30 days as confounders, decided a priori.
For children who had a second NFL sample (NFLtp2), the difference in NFL between the two time points (i.e., NFLtp2 minus NFLadm) was explored for associations between the baseline characteristics and for associations with the outcome measures.
All analyses were undertaken in R [30], using the packages RVAideMemoire and pROC for calculation of correlation coefficients and AUC ROC, respectively [31, 32]. A p value of < 0.05 was considered statistically significant.
Ethical approval for BASIC was granted by the East Midlands—Nottingham NRES Committee (ref 13-EM-0399). Informed consent was gained for BASIC study participation from families, with an option to withdraw consent at any stage of the study.
Results
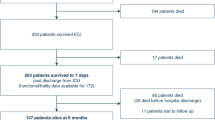
Six hundred seventy-four patients provided samples for the BASIC biobank. The median time from collection to processing of biobank samples was 22 h for admission samples and 3.7 h for second samples. One hundred twenty-four of 674 children had a 12-month follow-up. Adequate sample volume was available from 56/124 (45.2%) patients for NFL analysis (remaining samples following other assays). Three of 56 children did not have valid PedsQL scores. The median NFL value at admission (NFLadm) for the remaining 53 children was 17.0 pg/ml (IQR 5.8–28.3). One patient was a clear outlier with NFL > 2000 pg/ml. This patient had a mediastinal teratoma and given the possibility of neuronal tissue loss from within the teratoma, this patient was excluded from further analysis. Table 1 shows the baseline characteristics of the remaining 52 patients with samples (median NFLadm 17.0, IQR 5.8–28.1).
Fifty out of 52 (96.4%) patients were ventilated and 24/52 (46.1%) were on vaso-active drugs during their PICU admission. Eleven (21.1%) children needed continuous renal replacement therapy and 5 (9.6%) needed extracorporeal membrane oxygenation. Eighteen of 52 (34.6%) patients had abnormal neurological investigation during their PICU admission, either imaging (n = 10) or neurophysiology (n = 14).
Table 2 shows the univariable associations between the baseline characteristics and NFLadm. There was a negative correlation between SBP at admission and NFLadm. However, after adjusting for multiple comparisons, this does not meet the threshold for statistical significance. We also explored the distribution of NFLadm in various sub-groups (Fig. 1). Testing these statistically using the Mann–Whitney test, there were no significant differences in NFLadm for (i) those whose primary diagnosis was either a neurological disorder or trauma and those who had a different primary diagnosis (median 14.3 v 17.5 pg/ml, p value 0.84), (ii) those who had suffered a cardiac arrest pre-ICU admission and those who had not (median 18.3 v 17.0 pg/ml, p value 0.82), (iii) those with a neurological co-morbidity and those without (median 18.0 v 17.0 pg/ml, p value 0.45) and (iv) those who had a PCPC score > 1 (mild disability) at baseline and those who were reported normal (or had an unknown PCPC) (median 15.4 v 17.0 pg/ml, p value 0.94). Cardiac arrest pre-ICU ranged from the need for cardio-pulmonary resuscitation from 3 to 60 min (n = 7). While there was no correlation between the duration of cardiopulmonary resuscitation and NFLadm (Spearman correlation coefficient 0.07, 95% CI − 0.89, 0.89), the single patient with fixed and dilated pupils on admission did have a high NFLadm value (117 pg/ml).
Distribution of serum neurofilament light at admission (NFLadm) in the whole chort and different subgroups. The filled circles represent the cohort as shown across the x-axis: patients with neurological disease or trauma as the primary diagnosis, who had a cardiac arrest pre-ICU admission, those with a neurological co-morbidity, those with a Pediatric Cerebral Performance Category score > 1 (i.e. mild, moderate or severe disability, represented by darker shades of blue) at admission, abnormal brain imaging during the admission and abnormal neuro-physiology results during admission. When compared statistically, only NFLadm measurements for those with abnormal neuro-physiology results were higher than those in the cohort without (median 19.5 v 10.3 pg/ml, p value 0.03)
Primary outcome
The distribution of children categorised low or not low for PedsQL scores is shown in Table 3, along with the distribution of NFLadm according to the categorical outcomes. The AUC ROC for PedsQL was 0.52 (95% CI 0.34–0.69). Following logistic regression analysis, accounting for any co-morbidity and organ support free days at 30 days, NFLadm was not significantly associated with the PedsQL categories (NFLadm odds ratio 0.99, 95% CI 0.97, 1.02, p value 0.63; any co-morbidity 3.25, 95% CI 0.99, 11.62; organ support free days at 30 days 1.00, 95% CI 0.93, 1.08).
Secondary outcomes
In contrast to the distribution of the total PedsQL score, fewer children had a low physical score (14 low, 38 not low). NFLadm poorly predicted the physical component of the PedsQL score (AUC ROC 0.53, 95% CI 0.36–0.70).
Dichotomised CBCL scores were also poorly predicted by NFLadm, with an AUC ROC of 0.69 (95% CI 0.52–0.86). Unexpectedly, NFLadm was higher in children with CBCL scores below 93. NFLadm did not predict the response to the general questions about concerns, or whether the child was better, the same or worse (Table 3).
NFLadm was correlated with the length of invasive ventilation and length of stay in PICU and negatively correlated with the number of organ support free days at day 30 (Table 4). The AUC ROC for NFLadm predicting an abnormal neurological investigation on PICU was 0.67 (95% CI 0.52–0.83). NFLadm was a strong predictor of worsening of PCPC and POPC between PICU admission and discharge, but only 3 patients had worsening of PCPC or POPC (Table 4).
To evaluate how NFLadm performed compared to abnormal neurological investigations in PICU in predicting PedsQL score at 12 months, we compared the AUC ROC for each. Abnormal neurological investigations included acute or previously unknown changes on imaging (CT, MRI or cranial ultrasound in infants) or abnormal neurophysiology (seizures or encephalopathy on EEG or abnormal EMG or SSEPs). The AUC ROC for an abnormal neurological investigation (imaging, neurophysiology, or both) was 0.44 (95% CI 0.31–0.57). When compared to that of NFLadm, there was no significant difference in the AUC ROC (DeLong’s Z 0.73, p value 0.47). However, the NFLadm in those who had abnormal neurophysiology results was significantly higher than those who did not (median 19.5 v 10.3 pg/ml, p value 0.03), but this was not the case in those with abnormal brain imaging results (median 19.4 v 13.5 pg/ml, p value 0.26).
Nineteen of the 52 patients had a second NFL measurement (NFLtp2) (Fig. 2). The difference in NFL was not associated with any of the baseline characteristics (Supplemental Information Table 1). The AUC ROC for predicting the total PedsQL score was 0.51 (95% CI 0.23–0.79).
Distribution of serum neurofilament light at admission to the paediatric intensive care unit (NFLadm) and 24–48 h following admission (NFLtp2). The dashed lines indicate paired values: in 3/19 pairs NFL decreased between NFLadm and NFLtp2 (red) while in all others NFL increased between time points 1 and 2 (grey). The median difference in NFL between the time-points was was 14.5 (IQR 6.2–63.1)
Discussion
In this observational cohort study of NFL measurements in a heterogenous PICU population, NFL values were mostly low at admission, with a median of 17.0 pg/ml. NFLadm poorly predicted 12-month PedsQL scores but was correlated with the duration of PICU organ support and significantly associated with worsening of POPC/PCPC between PICU admission and discharge. In most cases, NFL increased within the first few days of PICU admission. The change in NFL did not predict outcomes in the small group of patients who had NFL values at two time points. However, our results are limited by the size of our cohort and at best should be considered exploratory.
One of the aims of this study was to describe the distribution of NFL in a heterogenous cohort of critically ill children following unplanned PICU admission. The largest description of NFL values in over 2000 healthy children by Geis et al. reported a mean NFL of 5.5 pg/ml (standard deviation 2.9 pg/ml) [33]. A smaller study of 292 healthy children defined a reference range of 3.5–16.6ng/L in children < 3 years and 2.1–13.9ng/L in those ≥ 3 years [34]. This was similar to the values reported by Kirschen in a much smaller cohort of healthy controls [15]. In comparison, Kirschen described higher NFL values post-cardiac arrest (median of 31.0 pg/ml, IQR 12.0–338.6). The mean value of NFL in autistic spectrum disease has been reported as 10.2 pg/ml [18], whereas in children with febrile illness, post-febrile seizures and post-epileptic seizures have median NFL values of 23.4, 21.7 and 17.7 pg/ml, respectively [17]. While the NFL values in our cohort are expectedly higher than healthy children, they are comparable to values with a range of illnesses from mild febrile illness to post-cardiac arrest. This may reflect the heterogeneity in the severity of illness of children admitted to PICU but also the timing of presentation.
The timing of the presentation may also explain the poor predictive value of the NFLadm for long-term outcomes. Wihesaari showed a serial increase in NFL values from admission every 24 to 72 h post-adult cardiac arrest, with an increasing predictive value [10]. The distribution of NFL over time is affected by two factors: the movement of NFL between the CSF and the blood compartments, and ongoing neuronal injury. In the 18 patients in whom paired NFL measurements at two time points could be analysed, NFL increased over the first 24–48 h of PICU admission in all but 3 cases. The magnitude of change however was not associated with outcome in this small sub-group.
We cannot comment on whether the rise in NFL between time points was due to the original neuronal injury, or secondary to the ongoing disease and treatment. The difference in NFL between the time points was greatest in those who had a cardiac arrest pre-ICU admission, those with a neurological disease or trauma as a primary diagnosis and those with neurological co-morbidities; however, any interpretation is impossible due to the sample size (hence no statistical significance, see Supplementary Information Table 1). PICU management may contribute to long-term outcomes, both by mediating the effect of the presenting illness, or by adding to neuronal injury through adverse events. This would be inadequately reflected in the NFL values at admission. Over half the children had co-morbidities. Our small sample size did not adequately allow us to account for these, despite multivariable analysis. While NFLadm has good predictive value for worsening of functional outcomes at PICU discharge, only 3 children had a worsening of their PCPC/POPC scores. These results have to be treated with caution.
There are several limitations to our study. The sample size limited the statistical power of any of the associations described. Based on the multi-variable analysis results (NFLadm p value 0.63, 49 degrees of freedom), we estimate that the power of our results is between 1 and 5%. Only samples from 8.5% of the children recruited to BASIC had available samples, survived and had a 12-month follow-up available. To look for systematic bias in those included, we compared the baseline characteristics and PICU outcomes in those with NFLadm measurements, those with follow-up data but without NFLadm measurements and those with neither (Supplementary Information Table 2). There are important differences evident from the descriptive statistics, with fewer children who had follow-up data with non-NFLadm measurements with neurological co-morbidities, and therefore normal PCPC at admission. More children in this cohort however had worsening of their PCPC between admission and discharge, possibly a reflection that they had a normal PCPC at admission. Non-recruitment of these children may have underestimated the predictive value of NFLadm. Serial measurements could have provided more information on the associations between disease trajectory, interventions and neuronal injury. There are possible reasons for small numbers with second samples: (i) some children recovered enough for their indwelling arterial or central venous lines to be removed prior to the second sampling time-point, and (ii) some parents consented to the use of already collected samples but declined the collection of further samples. Furthermore, serial measurements at later time points may have provided insight into children at risk of PICU-induced neurological injury such as critical illness polyneuropathy.
NFL is influenced by biological factors such as age and sex, and pathophysiological factors such as renal impairment [35]. Over one-fifth of children in our cohort needed renal replacement therapy—we did not account for any rise in NFL secondary to impaired clearance mechanisms, although this would have been difficult given that neuronal injury and kidney injury may share a common causal pathway. We also do not know whether extra-corporeal circuit binding may affect NFL measurements—this will need further evaluation.
The outcome measures were based on a 12-month follow-up. It may be possible that the effect of neuronal injury, particularly early in the course of critical illness may be better reflected at an earlier time point, e.g., at 3 or 6 months. The 12-month outcomes were measured remotely using tools that could be used in such a way: the tools may not be sensitive enough to detect the effects of neuronal damage from early in the course of critical illness. The outcome measures were all completed by parents and may be subject to reporting bias.
Our data provide a description of the distribution of NFL in critically ill children in PICU. To explore this further, we recommend a prospective, serial-sampling observational study to characterise the time-course NFL change in pediatric critical illness. If NFL does then correlate with meaningful clinical outcomes (such as death, worsening functional status of quality of life), then serial-sampling studies within randomised controlled interventional trials, where the intervention could potentially protect against neuronal loss (for example, blood pressure management or therapeutic hypothermia trials), could provide causal information regarding modifiable factors.
Conclusions
The distribution of NFL values measured early in critical illness is similar to those of other acute illnesses. We did not find an association between NFL and 12-month outcomes in this small heterogenous cohort of children admitted to PICU. There was an association between NFL and outcomes at PICU discharge, although the interpretation of this is limited by the sample size and low event rate.
Availability of data and materials
Data can be made available at request to the corresponding author.
References
Namachivayam P, Shann F, Shekerdemian L, Taylor A, van Sloten I, Delzoppo C, Daffey C, Butt W (2010) Three decades of pediatric intensive care: who was admitted, what happened in intensive care, and what happened afterward. Pediatr Crit Care Med 11(5):549–555. https://doi.org/10.1097/PCC.0b013e3181ce7427
PICANET (2014) Paediatric intensive care audit network: a decade of data (published September 2014): Universities of Leeds and Leicester. Available from https://www.picanet.org.uk/wp-content/uploads/sites/25/2018/05/PICANet_A_Decade_of_Data_2014_Annual_Report_Summary.pdf. Accessed 14 Mar 2022
PICANET (2019) Paediatric Intensive Care Audit Network Annual Report 2021 (published January 2022): Universities of Leeds and Leicester. Available from https://www.picanet.org.uk/wp-content/uploads/sites/25/2022/01/PICANet-2021-Annual-Report_v1.0-13Jan2022-2.pdf. Accessed 14 Mar 2022
Royer AS, Busari JO (2021) A systematic review of the impact of intensive care admissions on post discharge cognition in children. Eur J Pediatr 180(12):3443–3454. https://doi.org/10.1007/s00431-021-04145-5
Menon K, McNally JD, Zimmerman JJ, Agus MS, O’Hearn K, Watson RS, Wong HR, Duffett M, Wypij D, Choong K (2017) Primary outcome measures in pediatric septic shock trials: a systematic review. Pediatr Crit Care Med 18(3):e146–e154
Meert KL, Reeder R, Maddux AB, Banks R, Berg RA, Zuppa A, Newth CJ, Wessel D, Pollack MM, Hall MW, Quasney M, Sapru A, Carcillo JA, McQuillen PS, Mourani PM, Chima RS, Holubkov R, Sorenson S, Varni JW, McGalliard J, Haaland W, Whitlock KB, Dean JM, Zimmerman JJ, the Life After Pediatric Sepsis Evaluation (LAPSE) Investigators (2020) Trajectories and risk factors for altered physical and psychosocial health-related quality of life after pediatric community-acquired septic shock. Pediatr Crit Care Med. 21(10):869–878
Harbinson M, Zarshenas S, Cullen NK (2017) Long-term functional and psychosocial outcomes after hypoxic-ischemic brain injury: a case-controlled comparison to traumatic brain injury. PM R 9(12):1200–1207
Pek PP, Fan KC, Ong MEH, Luo N, Østbye T, Lim SL, Ho AF (2023) Determinants of health-related quality of life after out-of-hospital cardiac arrest (OHCA): a systematic review. Resuscitation 188:109794
Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, Barro C, Kappos L, Comabella M, Fazekas F, Petzold A, Blennow K, Zetterberg H, Kuhle J (2018) Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 14(10):577–589. https://doi.org/10.1038/s41582-018-0058-z
Wihersaari L, Ashton NJ, Reinikainen M, Jakkula P, Pettilä V, Hästbacka J, Tiainen M, Loisa P, Friberg H, Cronberg T, Blennow K, Zetterberg H, Skrifvars MB, COMACARE STUDY GROUP (2021) Neurofilament light as an outcome predictor after cardiac arrest: a post hoc analysis of the COMACARE trial. Intensive Care Med 47(1):39–48. https://doi.org/10.1007/s00134-020-06218-9
Moseby-Knappe M, Mattsson-Carlgren N, Stammet P, Backman S, Blennow K, Dankiewicz J, Friberg H, Hassager C, Horn J, Kjaergaard J, Lilja G, Rylander C, Ullén S, Undén J, Westhall E, Wise MP, Zetterberg H, Nielsen N, Cronberg T (2021) Serum markers of brain injury can predict good neurological outcome after out-of-hospital cardiac arrest. Intensive Care Med 47(9):984–994. https://doi.org/10.1007/s00134-021-06481-4
Goeral K, Hauck A, Atkinson A, Wagner MB, Pimpel B, Fuiko R, Klebermass-Schrehof K, Leppert D, Kuhle J, Berger A, Olischar M, Wellmann S (2021) Early life serum neurofilament dynamics predict neurodevelopmental outcome of preterm infants. J Neurol 268(7):2570–2577. https://doi.org/10.1007/s00415-021-10429-5
Sjöbom U, Hellström W, Löfqvist C, Nilsson AK, Holmström G, Pupp IH, Ley D, Blennow K, Zetterberg H, Sävman K, Hellström A (2021) Analysis of brain injury biomarker neurofilament light and neurodevelopmental outcomes and retinopathy of prematurity among preterm infants. JAMA Netw Open 4(4):e214138. https://doi.org/10.1001/jamanetworkopen.2021.4138
Shah DK, Ponnusamy V, Evanson J, Kapellou O, Ekitzidou G, Gupta N, Clarke P, Michael-Titus AT, Yip PK (2018) Raised plasma neurofilament light protein levels are associated with abnormal MRI outcomes in newborns undergoing therapeutic hypothermia. Front Neurol 5(9):86. https://doi.org/10.3389/fneur.2018.00086
Kirschen MP, Yehya N, Graham K, Kilbaugh T, Berg RA, Topjian A, Diaz-Arrastia R (2020) Circulating neurofilament light chain is associated with survival after pediatric cardiac arrest. Pediatr Crit Care Med 21(7):656–661
Fink EL, Kochanek PM, Panigrahy A, Beers SR, Berger RP, Bayir H, Pineda J, Newth C, Topjian AA, Press CA, Maddux AB, Willyerd F, Hunt EA, Siems A, Chung MG, Smith L, Wenger J, Doughty L, Diddle JW, Patregnani J, Piantino J, Walson KH, Balakrishnan B, Meyer MT, Friess S, Maloney D, Rubin P, Haller TL, Treble-Barna A, Wang C, Clark RRSB, Fabio A, Personalizing outcomes after child cardiac arrest (POCCA) investigators (2022) Association of Blood-Based Brain Injury Biomarker Concentrations With Outcomes After Pediatric Cardiac Arrest. JAMA Netw Open. 5(9):e2230518
Evers KS, Hügli M, Fouzas S, Kasser S, Pohl C, Stoecklin B, Bernasconi L, Kuhle J, Wellmann S (2020) Serum neurofilament levels in children with febrile seizures and in controls. Front Neurosci 29(14):579958. https://doi.org/10.3389/fnins.2020.579958
He WC, Zhang XJ, Zhang YQ, Zhang WJ (2020) Elevated serum neurofilament light chain in children autism spectrum disorder: a case control study. Neurotoxicology 80:87–92. https://doi.org/10.1016/j.neuro.2020.06.012
Giovannini G, Bedin R, Orlandi N, Turchi G, Cioclu MC, Biagioli N, Madrassi L, Pugnaghi M, Vaudano AE, Meletti S (2023) Neuro-glial degeneration in status epilepticus: exploring the role of serum levels of neurofilament light chains and S100B as prognostic biomarkers for short-term functional outcome. Epilepsy Behav 140:109131
AbdiIsse Y, Frikke-Schmidt R, Wiberg S, Grand J, Obling LER, Meyer ASP, Kjaergaard J, Hassager C, Meyer MAS (2023) Predicting poor neurological outcomes following out-of-hospital cardiac arrest using neuron-specific enolase and neurofilament light chain in patients with and without haemolysis. Eur Heart J Open. 3(4):oead078
Feinstein Y, Walker JC, Peters MJ, Nadel S, Pathan N, Edmonds N, Herberg J, Kaforou M, Wright V, Levin M, Ramnarayan P (2018) Cohort profile of the Biomarkers of Acute Serious Illness in Children (BASIC) study: a prospective multicentre cohort study in critically ill children. BMJ Open 8(11):e024729. https://doi.org/10.1136/bmjopen-2018-024729
Pulham RA, Wray J, Feinstein Y, Brown K, Pierce C, Nadel S, Pathan N, Garralda E, Ramnarayan P (2019) Feasibility and acceptability of methods to collect follow-up information from parents 12 months after their child’s emergency admission to pediatric intensive care. Pediatr Crit Care Med 20(4):e199–e207. https://doi.org/10.1097/PCC.0000000000001892
Weinhofer I, Rommer P, Zierfuss B, Altmann P, Foiani M, Heslegrave A, Zetterberg H, Gleiss A, Musolino PL, Gong Y, Forss-Petter S, Berger T, Eichler F, Aubourg P, Köhler W, Berger J (2021) Neurofilament light chain as a potential biomarker for monitoring neurodegeneration in X-linked adrenoleukodystrophy. Nat Commun 12(1):1816. https://doi.org/10.1038/s41467-021-22114-2
Varni JW, Seid M, Kurtin PS (2001) PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 39(8):800–12. https://doi.org/10.1097/00005650-200108000-00006
Varni JW, Limbers CA, Neighbors K, Schulz K, Lieu JE, Heffer RW, Tuzinkiewicz K, Mangione-Smith R, Zimmerman JJ, Alonso EM (2011) The PedsQL™ infant scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res 20(1):45–55. https://doi.org/10.1007/s11136-010-9730-5
Achenbach T (2009) M: the Achenbach System of Empirically Based Assessemnt (ASEBA): development, findings, theory, and applications. University of Vermont Research Center for Children, Youth, & Families, Burlington, VT
Varni JW, Burwinkle TM, Seid M, Skarr D (2003) The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 3(6):329–41. https://doi.org/10.1367/1539-4409(2003)003%3c0329:tpaapp%3e2.0.co;2
Cole TJ (1988) Fitting smooth centile curves to reference data. J Royal Stat Soc 151(3):385–406. https://doi.org/10.2307/2982992
Haque IU, Zaritsky AL (2007) Analysis of the evidence for the lower limit of systolic and mean arterial pressure in children. Pediatr Crit Care Med 8(2):138–144. https://doi.org/10.1097/01.PCC.0000257039.32593.DC
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (https://www.R-project.org/)
Hervé M (2020) VAideMemoire: testing and plotting procedures for biostatistics. R package version 0.9–78. https://CRAN.R-project.org/package=RVAideMemoire. Accessed 14 Mar 2022
Robin X, Turck N, Hainard A et al (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12:77. https://doi.org/10.1186/1471-2105-12-77
Geis T, Brandstetter S, Toncheva AA, Laub O, Leipold G, Wagner R, Kabesch M, Kasser S, Kuhle J, Wellmann S, CoKiBa Study Group (2021) Serum neurofilament light chain (sNfL) values in a large cross-sectional population of children with asymptomatic to moderate COVID-19. J Neurol. 268(11):3969–3974. https://doi.org/10.1007/s00415-021-10554-1
Schjørring ME, Parkner T, Knudsen CS, Tybirk L, Hviid CVB (2023) Neurofilament light chain: serum reference intervals in Danish children aged 0–17 years. Scand J Clin Lab Invest 26:1–5
Coppens S, Lehmann S, Hopley C, Hirtz C (2023) Neurofilament-light, a promising biomarker: analytical, metrological and clinical challenges. Int J Mol Sci 24(14):11624
Acknowledgements
We would like to acknowledge all members of the Children’s Acute Transport Service, clinical research team members at all three sites (Great Ormond Street Hospital for Children NHS Foundation Trust, Imperial College Healthcare NHS Trust and Addenbrookes Hospital, Cambridge University Hospitals NHS Trust), staff at the UK Dementia Research Institute at UCL and UCL GOS Institute of Child Health, and patients and families who participated in the BASIC study.
Funding
This work was supported by funding from the Great Ormond Street Hospital Children’s Charity and the UK Paediatric Critical Care Society. The HD-1 Analyzer was funded through a Wellcome Trust Multi-User Equipment Grant. Although not directly funded, the work was also supported by the National Institute for Health Research Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the UK Department of Health.
In addition, HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018–02532); the European Research Council (#681712); Swedish State Support for Clinical Research (#ALFGBG-720931); the Alzheimer Drug Discovery Foundation (ADDF); USA (#201809–2016862); the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21–831376-C, #ADSF-21–831381-C and #ADSF-21–831377-C); the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2019-0228); the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE); European Union Joint Program for Neurodegenerative Disorders (JPND2021-00694); and the UK Dementia Research Institute at UCL.
Author information
Authors and Affiliations
Contributions
PR devised and was the Chief Investigator for the BASIC biobank study; SR, MJP and PR designed the current study; GJ and AH analysed the samples; YF, SR and PR processed and analysed the data; JW designed and oversaw the follow-up data collection; all authors contributed to the drafting of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for BASIC was granted by the East Midlands—Nottingham NRES Committee (ref 13-EM-0399). Informed consent was gained for BASIC study participation from families, with an option to withdraw consent at any stage of the study.
Consent for publication
Informed consent was gained for BASIC study participation from families, with an option to withdraw consent at any stage of the study.
Competing interests
SR has undertaken consultancy work with Roche. HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Alector, Annexon, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Pinteon Therapeutics, Red Abbey Labs, Passage Bio, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen and Roche; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). No other authors have declared conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Digital Content Table 1.
Univariable analysis between baseline characteristics and change in serum neurofilament light values between admission to the paediatric intensive care unit (NFLadm) and 24-48 hours after enrolment (NFLtp2).
Additional file 2: Supplemental Digital Content Table 2.
Comparison of baseline characteristics and PICU outcomes in children who had NFLadm measured, had follow-up but no NFLadm sample and those who had neither NFLadm measured nor follow-up data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ray, S., Heslegrave, A., Jones, G. et al. Neurofilament light as a predictor of long- and short-term outcomes in critically ill children. Intensive Care Med. Paediatr. Neonatal 1, 20 (2023). https://doi.org/10.1007/s44253-023-00021-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44253-023-00021-2