Abstract
Biomedical devices provide a critical role in the healthcare system to positively impact patient well-being. This paper aims to provide the current classifications and subclassifications of hardware and software medical devices according to the Food and Drug Administration (FDA) guidelines. An overview of the FDA regulatory pathway for medical device development such as radiation-emitting electronic product verification, product classification database, Humanitarian Use Device (HUD), premarket approval, premarket notification and clearance, post-market surveillance, and reclassification is provided. Current advances and the advantages of implementing human factors engineering in biomedical device development to reduce the risk of user error, product recalls and effective safe use are discussed. This paper also provides a review of evolving topics such as the Internet of Things (IoT), software as medical devices, artificial intelligence (AI), machine learning (ML), mobile medical devices, and clinical decision software support systems. A comprehensive discussion of the first FDA-approved AI-medical device for the diagnosis of diabetic retinopathy is presented. Further, potential cybersecurity-related risks associated with software-driven AI/ML and IoT medical devices are discussed with an emphasis on government regulations. Futuristic trends in biomedical device development and their implications on patient care and the healthcare system are elucidated.












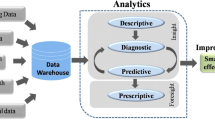
Copyright from [163]


Similar content being viewed by others
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
U.S. Food and Drugs Administration, Importing Medical Devices (2018), https://www.fda.gov/industry/importing-fda-regulated-products/importing-medical-devices#What is a medical device.
World Health Organization, Medical Devices. https://www.who.int/health-topics/medical-devices#tab=tab_1
C. Peña, K. Li, R. Felten, N. Ogden, M. Melkerson, An example of US Food and Drug Administration device regulation: medical devices indicated for use in acute ischemic stroke. Stroke 38(6), 1988–1992 (2007)
S.K. Gupta, Medical device regulations: a current perspective. J. Young Pharm. 8(1), 6 (2016)
S. Desai, S. Parupelli, Additive manufacturing (3D printing), in Maynard's Industrial and Systems Engineering Handbook, 6th ed. (Springer, Berlin, 2022)
S. Bose, S.F. Robertson, A. Bandyopadhyay, Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater. 66, 6–22 (2018)
M. Olowe, S.K. Parupelli, S. Desai, A review of 3D-printing of microneedles. Pharmaceutics 14(12), 2693 (2022)
E. Adarkwa, R. Kotoka, S. Desai, 3D printing of polymeric coatings on AZ31 Mg alloy substrate for corrosion protection of biomedical implants. Med. Devices Sensors 4(1), e10167 (2021)
G. Haeberle, S. Desai, Additive manufacturing (3D printing) of thermoform tooling. Int. J. Mech. Prod. Eng 7, 1–4 (2019)
A. Aljohani, S. Desai, 3D printing of porous scaffolds for medical applications. Am. J. Eng. Appl. Sci. 11(3), 1076–1085 (2018)
S.K. Parupelli, S. Desai, Understanding hybrid additive manufacturing of functional devices. Am. J. Eng. Appl. Sci. 10, 264–271 (2017). https://doi.org/10.3844/ajeassp.2017.264.271
F. Aldawood, S. Desai, Additive manufacturing of compensator devices for radiation therapy, in Proceedings of the 2020 IISE Annual Conference (2020)
J. McKenzie, S. Parupelli, D. Martin, S. Desai, Additive manufacturing of multiphase materials for electronics, in IIE Annual Conference. Proceedings, pp. 1133–1138 (2017)
L.J. Kelly, T. Jones, Medical device classification: focus on vascular access. Br. J. Nurs. 27(14), S14–S19 (2018). https://doi.org/10.12968/bjon.2018.27.14.S14
Centre for Devices & Radiological Health. FDA, Unique device identification and the EHR-types of medical devices and examples (2013), https://slideplayer.com/slide/1480438/. Accessed 13 July 2022
U.S. Food and Drug Administration-Products and Medical Procedures (2021), https://www.fda.gov/medical-devices/products-and-medical-procedures
A.N. Johnson, Medical Devices & MedTech Products (2020), https://angelanjohnson.com/medicaldevices/
FDA, US Food and Drugs Administration-Classify Your Medical Device (2018), https://www.fda.gov/medical-devices/overview-device-regulation/classify-your-medical-device
US Food and Drug Administration (FDA), Consumers (Medical Device), 2017. https://www.fda.gov/medical-devices/consumers-medical-devices/learn-if-medical-device-has-been-cleared-fda-marketing
A.V. Kaplan, D.S. Baim, J.J. Smith, D.A. Feigal, M. Simons, D. Jefferys, T.J. Fogarty, R.E. Kuntz, M.B. Leon, Medical device development: from prototype to regulatory approval. Circulation 109, 3068–3072 (2004)
L.H. Monsein, Primer on medical device regulation. Part II. Regulation of medical devices by the US Food and Drug Administration. Radiology 205, 10–18 (1997)
L. Keutzer, U.S.H. Simonsson, Medical device apps: an introduction to regulatory affairs for developers. JMIR mHealth uHealth 8(6), e17567 (2020)
B. Zhang, S.B. Shankara, J. Guo, H. Zhang, Pivotal clinical trials with patient-reported outcome measures in premarket approval applications for high-risk medical devices from 2005 to 2018: review, examples, and regulatory considerations. Contemp. Clin. Trials 116, 106757 (2022)
J. Li, M. Stachowski, Z. Zhang, Application of responsive polymers in implantable medical devices and biosensors, in Switchable and Responsive Surfaces and Materials for Biomedical Applications (Woodhead Publishing, Cambridge, 2015), pp. 259–298
R. Plowman et al., The rate and cost of hospital-acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed. J. Hosp. Infect. 47(3), 198–209 (2001). https://doi.org/10.1053/jhin.2000.0881
E.E. Bennett, J. VanBuren, S.L. Bratton, Presence of invasive devices and risks of healthcare-associated infections and sepsis. J. Pediatr. Intensive Care 7, 188–195 (2018)
N. Buetti et al., Strategies to prevent central line-associated bloodstream infections in acute-care hospitals: 2022 update. Infect. Control Hosp. Epidemiol. 43(5), 553–569 (2022)
R.B. Wilson, Y. Farooque, Risks and prevention of surgical site infection after hernia mesh repair and the predictive utility of ACS-NSQIP. J. Gastrointest. Surg. 26(4), 950–964 (2022)
R.J. Pratt et al., epic2: National evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J. Hosp. Infect. 65, S1–S59 (2007)
G. Flodgren, L.O. Conterno, A. Mayhew, O. Omar, C.R. Pereira, S. Shepperd, Interventions to improve professional adherence to guidelines for prevention of device‐related infections. Cochrane Database Syst. Rev. (3), CD006559 (2013)
D. Lebeaux, J.-M. Ghigo, C. Beloin, Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 78(3), 510–543 (2014)
L. Hall-Stoodley et al., Towards diagnostic guidelines for biofilm-associated infections. FEMS Immunol. Med. Microbiol. 65(2), 127–145 (2012)
M.K. Kasliwal, L.A. Tan, V.C. Traynelis, Infection with spinal instrumentation: review of pathogenesis, diagnosis, prevention, and management. Surg. Neurol. Int. 4(Suppl 5), S392 (2013)
L. Sangkum, G.L. Liu, L. Yu, H. Yan, A.D. Kaye, H. Liu, Minimally invasive or noninvasive cardiac output measurement: an update. J. Anesth. 30(3), 461–480 (2016). https://doi.org/10.1007/s00540-016-2154-9
C. Lin, Y. Tsai, Y. Lu, J. Yang, M. Chen, Application of a novel biosensor for salivary conductivity in detecting chronic kidney disease. Biosensors 12(3), 178 (2022). https://doi.org/10.3390/bios120301782022
W. Li, O. Auciello, R.N. Premnath, B. Kabius, Giant dielectric constant dominated by Maxwell–Wagner relaxation in Al2O3/TiO2 nanolaminates synthesized by atomic layer deposition. Appl. Phys. Lett. 96(16), 162907 (2010)
R. Magjarević, Non-invasive and minimally invasive medical devices. Report, May, pp. 10–12 (2017)
C.H. Chen, T.H. Tao, Y.H. Chou, Y.W. Chuang, T.B. Chen, Arteriovenous fistula flow dysfunction surveillance: early detection using pulse radar sensor and machine learning classification. Biosensors (2021). https://doi.org/10.3390/bios11090297
J.F. Mooney et al., Relative value of cystatin C and creatinine-based estimates of glomerular filtration rate in predicting long-term mortality after cardiac surgery: a cohort study. BMJ Open 9(9), e029379 (2019)
T.K. Chen, D.H. Knicely, M.E. Grams, Chronic kidney disease diagnosis and management: a review. JAMA 322(13), 1294–1304 (2019). https://doi.org/10.1001/jama.2019.14745
K.P. Bhatia et al., Consensus statement on the classification of tremors from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. 33(1), 75–87 (2018)
E.D. Louis, Tremor. Contin. Lifelong Learn. Neurol. 25(4), 959–975 (2019)
V. Srinivasan, V.K. Pamula, M.G. Pollack, R.B. Fair, Clinical diagnostics on human whole blood, plasma, serum, urine, saliva, sweat, and tears on a digital microfluidic platform, in Proc. MicroTAS, pp. 1287–1290 (2003)
G. Gaobotse, E. Mbunge, J. Batani, B. Muchemwa, Non-invasive smart implants in healthcare: Redefining healthcare services delivery through sensors and emerging digital health technologies. Sensors Int. 3, 100156 (2022). https://doi.org/10.1016/j.sintl.2022.100156
K. Dixit, S. Fardindoost, A. Ravishankara, N. Tasnim, M. Hoorfar, Exhaled breath analysis for diabetes diagnosis and monitoring: relevance, challenges and possibilities. Biosensors 11(12), 476 (2021)
K. Lee et al., Real-world outcomes of glucose sensor use in type 1 diabetes—findings from a large UK centre. Biosensors 11(11), 457 (2021)
Graphene patches over diabetes treatment (2016), https://www.sciencedirect.com/science/article/abs/pii/S1748013216301347?via%3Dihub
F.F. Franco, R.A. Hogg, L. Manjakkal, Cu2O-based electrochemical biosensor for non-invasive and portable glucose detection. Biosensors (2022). https://doi.org/10.3390/bios12030174
J. Kim, A.S. Campbell, B.E.-F. de Avila, J. Wang, Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37(4), 389–406 (2019)
R.J. Morrison et al., Regulatory considerations in the design and manufacturing of implantable 3D-printed medical devices. Clin. Transl. Sci. 8(5), 594–600 (2015). https://doi.org/10.1111/cts.12315
E. Adarkwa, A. Roy, J. Ohodnicki, B. Lee, P.N. Kumta, S. Desai, 3D printing of drug-eluting bioactive multifunctional coatings for orthopedic applications. Int. J. Bioprinting 9(2), 661 (2023)
S.K. Parupelli, S. Desai, Hybrid additive manufacturing (3D printing) and characterization of functionally gradient materials via in situ laser curing. Int. J. Adv. Manuf. Technol. 110, 543–556 (2020)
F.K. Aldawood, S.X. Chang, S. Desai, Design and manufacture of a high precision personalized electron bolus device for radiation therapy. Med. Devices Sensors 3(6), e10077 (2020)
G. Haeberle, S. Desai, Investigating rapid thermoform tooling via additive manufacturing (3D printing). Am. J. Appl. Sci 16, 238–243 (2019)
J. McKenzie, S. Desai, Investigating sintering mechanisms for additive manufacturing of conductive traces. Am. J. Eng. Appl. Sci. 11(2), 652–662 (2018)
S. Desai, B. Bidanda, P.J. Bártolo, Emerging trends in the applications of metallic and ceramic biomaterials, in Bio-Materials and Prototyping Applications in Medicine (Springer, Cham, 2021), pp. 1–17
S. Desai, M.R. Shankar, Emerging trends in polymers, composites, and nano biomaterial applications, in Bio-Materials and Prototyping Applications in Medicine (Springer, Cham, 2021), pp. 19–34
S. Desai, P. Gomes, Design for nano/micro manufacturing using a flexible decision making technique (AHP). J. Udyog Pragati 39(2), 18–25 (2015)
J. Perkins et al., Direct writing of polymeric coatings on magnesium alloy for tracheal stent applications. Ann. Biomed. Eng. 43, 1158–1165 (2015)
S. Desai, B. Harrison, Direct-writing of biomedia for drug delivery and tissue regeneration, in Printed Biomaterials: Novel Processing and Modeling Techniques for Medicine and Surgery, pp. 71–89 (2010)
S. Desai, M.R. Shankar, Polymers, composites and nano biomaterials: current and future developments, in Bio-Materials and Prototyping Applications in Medicine (Springer, Cham, 2008), pp. 15–26
S. Desai, B. Bidanda, Metallic and ceramic biomaterials: current and future developments, in Bio-Materials and Prototyping Applications in Medicine (Springer, Cham, 2008), pp. 1–14
P. Honigmann, N. Sharma, B. Okolo, U. Popp, B. Msallem, F.M. Thieringer, Patient-specific surgical implants made of 3D printed PEEK: material, technology, and scope of surgical application. Biomed. Res. Int. 2018, 4520636 (2018)
U.S. Food and Drugs Administration, Medical applications of 3D printing (2017), https://www.fda.gov/medical-devices/3d-printing-medical-devices/medical-applications-3d-printing
U.S. Food and Drugs Administration, Technical considerations for additive manufactured medical devices (2018), https://www.fda.gov/regulatory-information/search-fda-guidance-documents/technical-considerations-additive-manufactured-medical-devices
A.A. Raheem et al., A review on development of bio-inspired implants using 3D printing. Biomimetics 6(4), 65 (2021)
A.L. Jardini et al., Improvement in cranioplasty: advanced prosthesis biomanufacturing. Procedia CIRP 49, 203–208 (2016)
X. Chen, J.K. Possel, C. Wacongne, A.F. Van Ham, P.C. Klink, P.R. Roelfsema, 3D printing and modelling of customized implants and surgical guides for non-human primates. J. Neurosci. Methods 286, 38–55 (2017)
K. Phan, A. Sgro, M.M. Maharaj, P. D’Urso, R.J. Mobbs, Application of a 3D custom printed patient specific spinal implant for C1/2 arthrodesis. J. Spine Surg. 2(4), 314 (2016)
A. Dzian, J. Živčák, R. Penciak, R. Hudák, Implantation of a 3D-printed titanium sternum in a patient with a sternal tumor. World J. Surg. Oncol. 16, 1–4 (2018)
S. Gerke, B. Babic, T. Evgeniou, I.G. Cohen, The need for a system view to regulate artificial intelligence/machine learning-based software as medical device. npj Digit. Med. 3(1), 1–4 (2020). https://doi.org/10.1038/s41746-020-0262-2
S. Desai, M. Lovell, CFD analysis of a continuous inkjet print head for direct write fabrication, in ASME International Mechanical Engineering Congress and Exposition, vol. 43076, pp. 209–213 (2007)
J. Cordeiro, S. Desai, Process parameter studies of molecular dynamics models to control substrate wettability, in International Manufacturing Science and Engineering Conference, vol. 56826, p. V001T02A025 (2015)
J. Cordeiro, S. Desai, Exploring nano scale design space with molecular dynamics simulations, in IIE Annual Conference Proceedings, p. 856 (2015)
S. Desai, Methods and Apparatus for Manufacturing Micro-and/or Nano-Scale Features. Google Patents, 28 Nov 2013
S. Desai, M. Lovell, Modeling fluid–structure interaction in a direct write manufacturing process. J. Mater. Process. Technol. 212(10), 2031–2040 (2012)
E. Adarkwa, S. Desai, Scalable droplet based manufacturing using in-flight laser evaporation. J. Nanoeng. Nanomanuf. 6(2), 87–92 (2016)
S. Desai, M. Lovell, Multiphysics modeling of a piezoelectric bimorph disc in a direct write fabrication process, in ASME International Mechanical Engineering Congress and Exposition, vol. 42347, pp. 437–442 (2005)
S. Desai, M. Lovell, Coupled field analysis of a piezoelectric bimorph disc within a CIJ microfabrication process, in IIE Annual Conference. Proceedings, p. 1 (2006)
S. Murgu, H. Colt, Tracheobronchomalacia and excessive dynamic airway collapse. Clin. Chest Med. 34(3), 527–555 (2013)
S. Desai, J. Perkins, B.S. Harrison, J. Sankar, Understanding release kinetics of biopolymer drug delivery microcapsules for biomedical applications. Mater. Sci. Eng. B 168(1–3), 127–131 (2010)
S.K. Parupelli, A. Aljohani, S. Desai, S. Khanal, N. Bhattarai, Direct jet printing and characterization of calcium alginate microcapsules for biomedical applications, in IIE Annual Conference. Proceedings, pp. 300–305 (2019)
S. Desai, A. Moore, B. Harrison, J. Sankar, Understanding microdroplet formations for biomedical applications, in ASME International Mechanical Engineering Congress and Exposition, vol. 48760, pp. 119–123 (2008)
S. Desai, J. Sankar, A. Moore, B. Harrison, Biomanufacturing of microcapsules for drug delivery and tissue engineering applications, in IIE Annual Conference. Proceedings, p. 507 (2008)
J.L. Perkins, S. Desai, B. Harrison, J. Sankar, Understanding release kinetics of calcium alginate microcapsules using drop on demand inkjet printing, in ASME International Mechanical Engineering Congress and Exposition, vol. 43871, pp. 77–82 (2009)
S. Bose, K.D. Traxel, A.A. Vu, A. Bandyopadhyay, Clinical significance of three-dimensional printed biomaterials and biomedical devices. MRS Bull. 44(6), 494–504 (2019)
M. Zarek, N. Mansour, S. Shapira, D. Cohn, 4D printing of shape memory-based personalized endoluminal medical devices. Macromol. Rapid Commun. 38(2), 1–6 (2017). https://doi.org/10.1002/marc.201600628
J. Li, C. Wu, P.K. Chu, M. Gelinsky, 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 140, 100543 (2020)
A. Kirillova, R. Maxson, G. Stoychev, C.T. Gomillion, L. Ionov, 4D biofabrication using shape-morphing hydrogels. Adv. Mater. 29(46), 1703443 (2017)
H. Wei, Q. Zhang, Y. Yao, L. Liu, Y. Liu, J. Leng, Direct-write fabrication of 4D active shape-changing structures based on a shape memory polymer and its nanocomposite. ACS Appl. Mater. Interfaces 9(1), 876–883 (2017)
M.S. Cabrera, B. Sanders, O.J.G.M. Goor, A. Driessen-Mol, C.W.J. Oomens, F.P.T. Baaijens, Computationally designed 3D printed self-expandable polymer stents with biodegradation capacity for minimally invasive heart valve implantation: a proof-of-concept study. 3D Print. Addit. Manuf. 4(1), 19–29 (2017)
Y. Wang, H. Cui, T. Esworthy, D. Mei, Y. Wang, L.G. Zhang, Emerging 4D printing strategies for next-generation tissue regeneration and medical devices. Adv. Mater. 34(20), 2109198 (2022)
U.S. Food and Drugs Administration, Laser Products and Instruments (2023), https://www.fda.gov/radiation-emitting-products/home-business-and-entertainment-products/laser-products-and-instruments.
US Food and Drugs Administration, Radiation-Emitting Electronic Products (2018), https://www.fda.gov/industry/regulated-products/radiation-emitting-electronic-products. Accessed 8 Jul 2022
U.S. Food and Drugs Administration, Device Classification Panels (2018), https://www.fda.gov/medical-devices/classify-your-medical-device/device-classification-panels.
U.S. Food and Drugs Administration, Product Code Classification Database (2018), https://www.fda.gov/medical-devices/classify-your-medical-device/product-code-classification-database.
US Food and Drug Administration, Premarket Notification 510(k), March 13 (2020), https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/premarket-notification-510k. Accessed 8 Jul 2022
US Food and Drug Administration, Postmarket Requirements (Devices) (2018), https://www.fda.gov/medical-devices/device-advice-comprehensive-regulatory-assistance/postmarket-requirements-devices
U.S. Food and Drugs Administration, Reclassification (2021), https://www.fda.gov/about-fda/cdrh-transparency/reclassification
U.S. Food and Drugs Administration, Medical Device Accessories (2020), https://www.fda.gov/medical-devices/classify-your-medical-device/medical-device-accessories
U.S. Food and Drugs Administration, CFR—Code of Federal Regulations Title 21 (2023), https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=820.3
W. Gilmore, The User-Computer Interface in Process Control: A Human Factors Engineering Handbook (Elsevier, Amsterdam, 2012)
R. Gagnon et al., A user-centered evaluation of three intravenous infusion pumps, in Proceedings of the Human Factors and Ergonomics Society Annual Meeting, vol. 48, no. 15, pp. 1773–1777 (2004)
L. Lin, K.J. Vicente, D.J. Doyle, Patient safety, potential adverse drug events, and medical device design: a human factors engineering approach. J. Biomed. Inform. 34(4), 274–284 (2001)
D. Sawyer, K.J. Aziz, C.L. Backinger, E.T. Beers, A. Lowery, S.M. Sykes, An introduction to human factors in medical devices, U.S. Department of Health and Human Services Public Health Service Food and Drug Administration Center for Devices and Radiological Health, p. 55 (1996)
N.E. Schaeffer, The role of human factors in the design and development of an insulin pump. J. Diabetes Sci. Technol. 6(2), 260–264 (2012). https://doi.org/10.1177/193229681200600208
J.-E. Kim, L. Kessler, Z. McCauley, I. Niiyama, L.N. Boyle, Human factors considerations in designing a personalized mobile dialysis device: an interview study. Appl. Ergon. 85, 103003 (2020)
R. North, C. Pospisil, R.J. Clukey, C.G. Parkin, Impact of human factors testing on medical device design: validation of an automated CGM sensor applicator. J. Diabetes Sci. Technol. 13(5), 949–953 (2019). https://doi.org/10.1177/1932296819831071
A.L. Cassano, Applying human factors to the procurement of electrosurgical medical devices: a case study. Proceedings of the Human Factors and Ergonomics Society Annual Meeting 47(15), 1815–1819 (2003)
C.J. Vincent, Y. Li, A. Blandford, Integration of human factors and ergonomics during medical device design and development: it’s all about communication. Appl. Ergon. 45(3), 413–419 (2014). https://doi.org/10.1016/j.apergo.2013.05.009
G. Ginsburg, Human factors engineering: a tool for medical device evaluation in hospital procurement decision-making. J. Biomed. Inform. 38(3), 213–219 (2005). https://doi.org/10.1016/j.jbi.2004.11.008
J. Nielsen, R. Molich, Heuristic evaluation of user interfaces, in Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, pp. 249–256 (1990)
B. Schneiderman, Designing the User Interface. Strategies for Effective Human–Computer Interaction (Addison-Wesley, Hoboken, 1992)
M.-H. Maras, Internet of Things: security and privacy implications. Int. Data Priv. Law 5(2), 99 (2015)
A. Chacko, T. Hayajneh, Security and privacy issues with IoT in healthcare. EAI Endorsed Trans. Pervasive Heal. Technol. 4(14), 1–8 (2018). https://doi.org/10.4108/eai.13-7-2018.155079
F.Z. Amara, M. Hemam, M. Djezzar, M. Maimor, Semantic web and Internet of Things: challenges, applications and perspectives. J. ICT Stand. 10(2), 261–292 (2022)
B. Pradhan, S. Bhattacharyya, K. Pal, IoT-based applications in healthcare devices. J. Healthc. Eng. 2021, 6632599 (2021). https://doi.org/10.1155/2021/6632599
L.M. Dang, M.J. Piran, D. Han, K. Min, H. Moon, A survey on internet of things and cloud computing for healthcare. Electronics 8(7), 768 (2019)
R. Hughes, Patient Safety and Quality: An Evidence-Based Handbook for Nurses (Agency for Healthcare Research and Quality, Rockville, 2008)
S.E. Bibri, Ethical implications of AMI and the IoT: risks to privacy, security, and trust, and prospective technological safeguards, in The Shaping of Ambient Intelligence and the Internet of Things (Atlantis Press, Paris, 2015), pp. 217–238
D.S. Punithavathani, K. Sujatha, J.M. Jain, Surveillance of anomaly and misuse in critical networks to counter insider threats using computational intelligence. Cluster Comput. 18(1), 435–451 (2015)
B. Altubaishe, S. Desai, Multicriteria decision making in supply chain management using FMEA and hybrid AHP-PROMETHEE algorithms. Sensors 23(8), 4041 (2023)
U.S. Food and Drugs Administration, Software as a medical device (SaMD) (2018), https://www.fda.gov/medical-devices/digital-health-center-excellence/software-medical-device-samd
US Food and Drugs Administration, Policy for device software functions and mobile medical applications. Guidance for Industry and Food and Drug Administration Staff (2019), https://www.fda.gov/media/80958/download
U.S. Food and Drugs Administration, Digital Health Criteria (2018), https://www.fda.gov/medical-devices/digital-health-center-excellence/digital-health-criteria
International Medical Device Regulators Forum, Software as a medical device: possible framework for risk categorization and corresponding considerations (2014), http://www.imdrf.org/docs/imdrf/final/technical/imdrf-tech-140918-samd-framework-risk-categorization-141013.pdf
US Food and Drugs Administration, What are examples of Software as a Medical Device? (2017) https://www.fda.gov/medical-devices/software-medical-device-samd/what-are-examples-software-medical-device
Y. Fang, D. Liu, Z. Jiang, H. Wang, Monitoring of sleep breathing states based on audio sensor utilizing Mel-Scale features in home healthcare. J. Healthc. Eng. 2023, 6197564 (2023). https://doi.org/10.1155/2023/6197564
E.F. Camargos, F.M. Louzada, O.T. Nóbrega, Wrist actigraphy for measuring sleep in intervention studies with Alzheimer’s disease patients: application, usefulness, and challenges. Sleep Med. Rev. 17(6), 475–488 (2013)
S.-J. Zhou et al., Measuring sleep stages and screening for obstructive sleep apnea with a wearable multi-sensor system in comparison to polysomnography. Nat. Sci. Sleep 15, 353–362 (2023). https://doi.org/10.2147/NSS.S406359
V. Patel, A. Chesmore, C.M. Legner, S. Pandey, Trends in workplace wearable technologies and connected-worker solutions for next-generation occupational safety, health, and productivity. Adv. Intell. Syst. 4(1), 2100099 (2022)
Y. Xue, A review on intelligent wearables: Uses and risks. Hum. Behav. Emerg. Technol. 1(4), 287–294 (2019)
M.C. Schall Jr., R.F. Sesek, L.A. Cavuoto, Barriers to the adoption of wearable sensors in the workplace: a survey of occupational safety and health professionals. Hum. Factors 60(3), 351–362 (2018)
J. Kim et al., Simultaneous monitoring of sweat and interstitial fluid using a single wearable biosensor platform. Adv. Sci. 5(10), 1800880 (2018)
A. Koh et al., A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 8(366), 1-366ra165 (2016)
J. Kim et al., Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst 139(7), 1632–1636 (2014)
F. Güder et al., Paper-based electrical respiration sensor. Angew. Chem. Int. Ed. 55(19), 5727–5732 (2016)
M. Padash, C. Enz, S. Carrara, Microfluidics by additive manufacturing for wearable biosensors: a review. Sensors 20(15), 4236 (2020)
U.S. Food and Drugs Administration, Wireless Medical Device (2018), https://www.fda.gov/medical-devices/digital-health-center-excellence/wireless-medical-devices
P. Hamet, J. Tremblay, Artificial intelligence in medicine. Metabolism 69, S36–S40 (2017). https://doi.org/10.1016/j.metabol.2017.01.011
L. Monostori, in Artificial Intelligence BT, in CIRP Encyclopedia of Production Engineering, ed. by L. Laperrière, G. Reinhart (Springer, Berlin, 2014), pp. 47–50
F. Jiang et al., Artificial intelligence in healthcare: past, present and future. Stroke Vasc. Neurol. 2(4), 230–243 (2017)
A.M. Darcy, A.K. Louie, L.W. Roberts, Machine learning and the profession of medicine. JAMA 315(6), 551–552 (2016)
H.J. Murff et al., Automated identification of postoperative complications within an electronic medical record using natural language processing. JAMA 306(8), 848–855 (2011)
U.S. Food and Drugs Administration, Artificial Intelligence and Machine Learning in Software as a Medical Device (2021), https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-software-medical-device
Y. Xu et al., Artificial intelligence: a powerful paradigm for scientific research. Innovations 2(4), 100179 (2021). https://doi.org/10.1016/j.xinn.2021.100179
M. Ogunsanya, S. Desai, Predictive modeling of additive manufacturing process using deep learning algorithm, in IIE Annual Conference. Proceedings, pp. 1–6 (2022)
H. Elhoone, T. Zhang, M. Anwar, S. Desai, Cyber-based design for additive manufacturing using artificial neural networks for Industry 4.0. Int. J. Prod. Res. 58(9), 2841–2861 (2020)
T. Akter, S. Desai, Developing a predictive model for nanoimprint lithography using artificial neural networks. Mater. Des. 160, 836–848 (2018)
S. Desai, C. Dean, Y. Desai, Cyber-enabled concurrent material and process selection in a flexible design for manufacture paradigm. Int. J. Adv. Manuf. Technol. 97(5–8), 1719–1731 (2018)
K.W. Johnson et al., Artificial intelligence in cardiology. J. Am. Coll. Cardiol. 71(23), 2668–2679 (2018). https://doi.org/10.1016/j.jacc.2018.03.521
A.-M. Singeap, C. Stanciu, A. Trifan, Capsule endoscopy: the road ahead. World J. Gastroenterol. 22(1), 369 (2016)
B. Sushma, P. Aparna, Recent developments in wireless capsule endoscopy imaging: Compression and summarization techniques. Comput. Biol. Med. 149, 106087 (2022). https://doi.org/10.1016/j.compbiomed.2022.106087
D. Nikolayev, M. Zhadobov, R. Sauleau, P. Karban, Antennas for ingestible capsule telemetry, in Advances in Body-Centric Wireless Communication: Applications and State-of-the-Art (IET, London, 2016), pp. 143–186
P.P. Stanich, B. Kleinman, K. Betkerur, N. Mehta Oza, K. Porter, M.M. Meyer, Video capsule endoscopy is successful and effective in outpatients with implantable cardiac devices. Dig. Endosc. 26(6), 726–730 (2014)
I. Tziortziotis, F.-M. Laskaratos, S. Coda, Role of artificial intelligence in video capsule endoscopy. Diagnostics 11(7), 1192 (2021)
G.S. Raju, L. Gerson, A. Das, B. Lewis, American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. Gastroenterology 133(5), 1697–1717 (2007)
H. Bay, A. Ess, T. Tuytelaars, L. Van Gool, Speeded-Up Robust Features (SURF). Comput. Vis. Image Underst. 110(3), 346–359 (2008). https://doi.org/10.1016/j.cviu.2007.09.014
D.K. Iakovidis, S. Tsevas, A. Polydorou, Reduction of capsule endoscopy reading times by unsupervised image mining. Comput. Med. Imaging Graph. 34(6), 471–478 (2010). https://doi.org/10.1016/j.compmedimag.2009.11.005
H.-G. Lee, M.-K. Choi, B.-S. Shin, S.-C. Lee, Reducing redundancy in wireless capsule endoscopy videos. Comput. Biol. Med. 43(6), 670–682 (2013). https://doi.org/10.1016/j.compbiomed.2013.02.009
S.C. Payne, J.B. Furness, M.J. Stebbing, Bioelectric neuromodulation for gastrointestinal disorders: effectiveness and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 16(2), 89–105 (2019)
D. Miley, L.B. Machado, C. Condo, A.E. Jergens, K.-J. Yoon, S. Pandey, Video capsule endoscopy and ingestible electronics: emerging trends in sensors, circuits, materials, telemetry, optics, and rapid reading software. Adv. Devices Instrum. (2021). https://doi.org/10.34133/2021/9854040
T. Nakamura, Capsule endoscopy in Japan. Dig. Endosc. 34, 76–78 (2022)
J. Park et al., Recent development of computer vision technology to improve capsule endoscopy. Clin. Endosc. 52(4), 328–333 (2019)
X. Jia, M.Q.-H. Meng, Gastrointestinal bleeding detection in wireless capsule endoscopy images using handcrafted and CNN features, in 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), pp. 3154–3157 (2017). https://doi.org/10.1109/EMBC.2017.8037526
Y. Yuan, M.Q. Meng, Deep learning for polyp recognition in wireless capsule endoscopy images. Med. Phys. 44(4), 1379–1389 (2017)
D.K. Iakovidis, A. Koulaouzidis, Software for enhanced video capsule endoscopy: challenges for essential progress. Nat. Rev. Gastroenterol. Hepatol. 12(3), 172–186 (2015). https://doi.org/10.1038/nrgastro.2015.13
Z. Ding et al., Gastroenterologist-level identification of small-bowel diseases and normal variants by capsule endoscopy using a deep-learning model. Gastroenterology 157(4), 1044–1054 (2019)
T. Aoki et al., Clinical usefulness of a deep learning-based system as the first screening on small-bowel capsule endoscopy reading. Dig. Endosc. 32(4), 585–591 (2020)
G. Ciuti et al., Frontiers of robotic endoscopic capsules: a review. J. Micro-bio Robot. 11, 1–18 (2016)
J.C. Norton et al., Intelligent magnetic manipulation for gastrointestinal ultrasound. Sci. Robot. 4(31), eaav7725 (2019)
J. Min, Y. Yang, Z. Wu, W. Gao, Robotics in the gut. Adv. Ther. 3(4), 1900125 (2020)
U.S. Food and Drugs Administration, Ingestible telemetric gastrointestinal capsule imaging system—final class ii special controls guidance document for industry and FDA (2018), https://www.fda.gov/medical-devices/guidance-documents-medical-devices-and-radiation-emitting-products/ingestible-telemetric-gastrointestinal-capsule-imaging-system-final-class-ii-special-controls
B. Prabhakar, R.K. Singh, K.S. Yadav, Artificial intelligence (AI) impacting diagnosis of glaucoma and understanding the regulatory aspects of AI-based software as medical device. Comput. Med. Imaging Graph. 87, 101818 (2021). https://doi.org/10.1016/j.compmedimag.2020.101818
X. Zhang et al., Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA 304(6), 649–656 (2010)
Cruise Ophthalmic, Portable eye fundus camera Digital Retinal Scan Photography. https://www.aliexpress.com/item/3256801216401596.html?gatewayAdapt=4itemAdapt
Digital Diagnosis-AI the right way. https://www.digitaldiagnostics.com/products/eye-disease/idx-dr/?gclid=CjwKCAjw46CVBhB1EiwAgy6M4hNGGgKBv1kg0j_9nkFxQHp5UqBclhg-5WyP8KGTFVOLrpYWTGp7fRoCuPIQAvD_BwE
F. Mahgoub, Here’s How the FDA Approval of IDx-DR Will Impact Patients (2018), https://introwellness.com/eyes/idx-dr/
E.H. Nepal, Review of 3nethra Classic Digital Non-mydriatic Fundus Camera (2021), https://www.eyehealthnepal.com/3nethra-classic-fundus-camera/
L. Tsang et al., The impact of artificial intelligence on medical innovation in the European Union and United States. Intellect. Prop. Technol. Law J. 29(8), 3–12 (2017)
U.S. Food and Drugs Administration, Device Software Functions Including Mobile Medical Applications (2022), https://www.fda.gov/medical-devices/digital-health-center-excellence/device-software-functions-including-mobile-medical-applications
U.S. Food and Drugs Administration, Medical Device Data Systems (2019), https://www.fda.gov/medical-devices/general-hospital-devices-and-supplies/medical-device-data-systems
G. Freckmann et al., Insulin pump therapy for patients with type 2 diabetes mellitus: evidence, current barriers, and new technologies. J. Diabetes Sci. Technol. 15(4), 901–915 (2021)
N.J. Wimmer et al., Assessing the cost burden of United States FDA-mandated post-approval studies for medical devices. J. Health Care Finance 2016(Spec FEATURES) (2016)
Acknowledgements
The authors acknowledge the funding from the National Science Foundation (NSF) #1663128, #2100739, #2100850, #2200538. We would like to thank the Center of Excellence in Product Design and Advanced Manufacturing (CEPDAM), North Carolina A & T State University.
Funding
The authors acknowledge the funding from the National Science Foundation (NSF) #1663128, #2100739, #2100850, #2200538.
Author information
Authors and Affiliations
Contributions
All authors contributed to the composition of the manuscript. The final version of the manuscript has been approved by all authors.
Corresponding author
Ethics declarations
Competing interests
There are no competing interests for this review article.
Ethical Approval
All the information presented is cited appropriately and relevant literature in support of the claims made.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tettey, F., Parupelli, S.K. & Desai, S. A Review of Biomedical Devices: Classification, Regulatory Guidelines, Human Factors, Software as a Medical Device, and Cybersecurity. Biomedical Materials & Devices 2, 316–341 (2024). https://doi.org/10.1007/s44174-023-00113-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44174-023-00113-9