Abstract
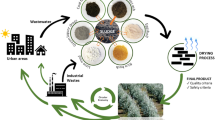
Slurries are a problem for the food-producing industry. They are comprised of solid and liquid wastes which must be precipitated and packed in polymers, and then disposed of in special landfills. A package of chemical substances (TCAS-CATA) has been developed to control the smell of this type of material. However, questions arose as to whether it would have any effect on nutrient bioavailability after eliminating the odors. To this end, slurries from animal processing plants were treated with TCAS-CATA in different conditions, and the parameters of this resulting solution were measured. Our results indicate that the catalyst can increase 100% nitrogenated compound concentration (400 mg/L of nitrites and 250 mg/L of Nitrate). This nutrient liberation depends on the slurry source, i.e., slurries with shells generate fewer nitrogenated compounds, but deliver more calcium to the solution. The solution also generates germination in some seed types and can stimulate development in some types of plants. Finally, the catalyst reduces odor 100% without reducing the capacity of the slurry to deliver nutrients. Our data suggest that TCAS-CATA may be catalyzing the bioavailability of the compound from the slurry without requiring microbiological processes, which will reduce odor and permit the use of this slurry as a biological remediation, similar to what is achieved in composting systems, but without the associated thermophilic processes. Future studies will allow for more knowledge of the mechanism and handling industrial material for agricultural processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Obtaining nutrients for crops is a problem in agricultural processes [1]. Nitrogen sources currently come primarily from nitrogenated compounds, which must be incorporated into cultures, and which carry high costs [2]. New sources allowing for use of organic wastes appear to be sources for delivering nutrients to the soil, but the principal model is composting which presents time and odor problems throughout the process [3, 4]. Composting, the main model for managing organic wastes, helps to generate soil with a high degree of organic material for agricultural purposes [5, 6]. Composting is a strategy to reduce organic garbage volumes, thereby aiding in organic waste problems at both the industrial and household levels [7]. In general terms, composting is a rather complex biochemical process which concentrates the decomposition process, allowing for more control of its variables and achieving better efficiency. However, it has various entry and process requirements which limit its action, as well as generating a type of product which is not always completely used and requires various biological components [8]. Composting is a special pathway of decomposition, which is the way that organic material degrades into simple molecules, liberating organic and inorganic material into the environment. The decomposition (stabilization) process of organic material by biological action is an intrinsic part of life, and fundamental to ecosystems. In this process, the presence of necrobiota (Bacteria, fungi, protozoa, and others) corresponds to saprophytic organisms which begin the process by releasing molecules that feed the following food chain links [9]. Composting is mainly a strategy to perform controlled decomposition and generate soil, but the process presents a few problem in the field for massive application [10].
The proposal is to evaluate a compound with the capacity to catalyze organic material, allowing for the liberation of its nutrients, and making it useful to improve agricultural soils. Catalysis in chemistry is defined as the capacity to reduce the energy needed to transform a substrate into a product. This phenomenon is generated by molecules with enzymatic capacities, allowing for the generation of a typical reaction curve with constants defined in the Michaelis-Menten equation [11]. A molecule with this type of reaction can be considered an enzymatic catalyst, making it possible to reduce energy, and therefore reaction time [11]. This is the principle of composting, which accelerates biochemical processes and reactions via closed conditions [12]. Within these circumstances, it has been indicated that material generated within organic systems can be discharged as organic fertilizer, which has certain characteristics ranging from its organic material content to its humidity and physiochemical properties. This system can be used in farming as a fertilizer and is an alternative to composting which takes less time but has lower nutritional value according to its origin sources [13, 14]. TCAS-CATA compound development by the TEQUIA corporation was evaluated in the laboratory, measuring organic compounds released from organic matter exposed to the compound to produce organic nutrients, reduce the odor and promote germination, be suitable for future farming use from various organic materials and without generating strong odors or needing source usage restrictions, and incorporate industrial waste into this process.
2 Methods
2.1 TCAS-CATA
TCAS-CATA is a product under patent register and product register in national law. It is a complex mixture of chemical compound and one anionic surfactant Sodium Dodecylbenzene Sulfonate SDBS as an active principle, reported to the national environment service in Chile.
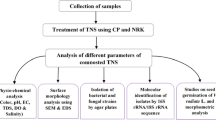
2.2 Obtaining samples
Slurry samples were used from various food production companies. The samples are blot water, mud (a mixture of water, blood, and meat from Tattersal, Osorno, Chile) and meat (bovine meat rest from Tattersal, Osorno, Chile). We also used seafood waste (bivalves, crustacean and eggshell, from St Andrews, Chonchi, Chile). The slurry collected from the different origins presented low concentrations of nitrogen, phosphorus, and calcium. The slurry presents 60% of humidity and pH 6.7. There is no presence of pathogen, fungi or other microorganism. The general odor description of the slurry is a noxious stench, with the presence of ammonia gas and sulfuric acid gas. The sample was preserved at low temperature, taken to the laboratory, and treated with TCAS CATA™ solution at different of dilution V/V to 0.1% to 5% prior to continuous shaking, in a 1:1 proportion with the compound at different dilutions for 0 to 180 min. Samples for biochemistry and odor observation were prepared by taking 20 ml of the samples in a beaker following different catalyzing time lengths from 0 to 14 hrs and carrying out parameter measurements.
2.3 Odor evaluation
Independent observers were asked to indicate unpleasantness on a subjective scale from 0 to 4. This was done before treatment, after applying the treatment and in samples over the same time without treatment, all according to the considerations of the NCh3190 standard regarding static or terrain observations of environmental odor evaluation.
2.4 Nitrite and nitrate measurement
A Hanna spectrophotometer, instrument model HI83399–02 was used. For the measurement a series of Hanna reactive agents was used, which follow an adaptation of the Ferrous Sulfate Method where the reaction between the nitrite and the reactive causes a color change in the sample. For the nitrate, the cadmium reduction adaptation method was used. The reaction between nitrate-nitrogen and the reactive caused an amber tone in the sample measured at 700 nm. In both cases, color intensity was determined by a compatible photometer, and the concentration will be shown in mg/L (ppm) of nitrite or nitrate.
2.5 Measurement of nitrogen, potassium and phosphate
A Hanna spectrometer, instrument model HI83399–02 was used. For the measurement biochemical techniques recommended by Hanna Instruments were used according to EPA norms. For potassium modification of the turbidimetry techniques based on tetraphenylborate was used, measured at 620 nm. For nitrogen chromatographic acid techniques were used, as indirect nitrite and nitrate measures made to 700 nm. For phosphate, a method adapted from ascorbic acid was used, indirectly estimating the phosphorus concentration 550 nm. In all cases color intensity is determined via a compatible photometer and the concentration will be shown in mg/L (ppm) of the various metabolites.
2.6 Measurement of calcium
A Hanna spectrometer, instrument model HI83399–02 was used. For the measurement, biochemical techniques recommended by Hanna Instruments according to EPA norms were used.
2.7 Measurement germination and sprout
Seeds of Solanum lycopersicum were taken for research. The seeds were put into a solid matrix of soil. In case of S. lycopersicum germination, control soil was used and mixed with catalysts from various sources, mud, meat and blood water and was observed the day the seeds germinated. Other seeds were observed for the presence of a sprout and were monitored temporarily, to see their growth. Irrigation was used with control water and mixed with catalyzed supernatant in increasing concentrations V / V (0.1–5%). The growth in centimeters of the stem of the germinated seed was observed day by day.
2.8 Data analysis
All the experiments were made in three replicas, and 6 independent experiments were made. Data were graphed and treated with statistical normality tests, a two-path ANOVA test with a Bonferroni test, using the Prism Gradphad program (version 5). Data are presented on average ± SEM, with p > 0.5 being considered significant.
3 Results
3.1 Time measurement of nitrite and nitrate concentration
The use of nitrogenated molecules has been described to evaluate compost and soil quality. The observation of minerals such as calcium also makes it possible to obtain information about organic fertilizer quality [15]. Figure 1A shows a progress curve for slurry catalyzed with a 1% TCAS-CATA solution, observing changes in the nitrite and nitrate concentration as a function of the time curve. Figure 2A shows free calcium concentration in the sample catalyzed with a 1% TCAS-CATA solution as a function of time. Both slurries are from slaughterhouse production and showed significant levels after 60 minutes of channeling.
Release of compound. Fig. A shows a graph of points on a time-progress curve of the nitrogenous compound concentrations obtained from a mud treated with TCAS-CATA at 1%. In B, a graph of points of a time-progress curve of the calcium concentration obtained from a sludge with eggshell treated with TCAS-CATA at 1%. The points are representative of the average ± SEM, from 6 independent experiments
Effect of origin in release compounds. Fig. A (sample bar graph) different conditions in absence or presence of TCAS 1% for 60 min. The concentration of calcium generated in solution B (sample bar graph) different conditions in absence or presence of TCAS 1% for 60 min. The concentration of nitrites generated in the solution. The bars are representative of the average ± SEM, of 3 independent experiments. The asterisk represents a p < 0.05 (ANOVA)
3.2 Parameter measurement for calcium and nitrites according to organic material source
It can be indicated that slurries from various sources will have diverse nutritional profiles. In this line, diverse production processes were used, taking their waste products and catalyzing them for 60 minutes with a 1% TCAS-CATA solution. Figure 2A shows the concentration of free calcium from slurry sources, both marine and bovine. A differential increase of calcium appears, with significantly more abundance from samples of eggshells and bivalve shells. Figure 2B shows nitrite concentration from various sources and indicates that slurry is significantly higher in nitrite concentration. This may indicate that samples could be mixed, generating catalyzed material with greater nutritional value for soils.
3.3 Effect of temperature on nitrite and nitrate concentration
The presence of nitrogenated compounds in the decomposition process depends on temperature for bacteria development [16]. The data seem to suggest that TCAS-CATA can generate this catalysis, but it must be observed how this variable affects the compound. Figure 3A shows a progress curve in nitrite and nitrate concentrations in a slaughterhouse slurry, without catalyst. We can observe that room temperature samples (RM) without the catalyst achieve nitrogenated compound generation, while this is absent at low temperatures. Figure 3B shows the same progress curve, but with the presence of 1% TCAS-CATA. Here we see that in all temperature conditions nitrogenated compounds can be released, being greater than RM when comparing catalysts between themselves. This suggests that while there is a release of nitrogenated compounds at RM without the catalyst, their efficiency is greater with the catalyst, indicating quicker nutrient release from the industrial slurry.
Nutrient release kinetics. Fig. A shows a graph of points on a time-progress curve of the nitrogen compound concentrations obtained from a mud kept under different temperature conditions. B is a graph of points on a time-progress curve of the nitrogenous compound concentrations obtained from a mud kept in different temperature conditions TCAS-CATA at 1%. The points are representative of the average ± SEM, from 6 independent experiments
3.4 Evaluation of sample odors, concentration effect
Many of the problems with using organic material in farming arise from the release of unpleasant odors, due to the anaerobic decomposition process [16]. The composting process aims to accelerate this process in closed conditions and reduce the problem [5]. These facts suggest that TCAS-CATA generates material release without a fermentation process, and thus without loathsome odors. Figure 4A shows a graph of odor evaluation in independent observers of a slaughterhouse slurry catalyzed with TCAS-CATA and shows the effect of odor appreciation at different concentrations and times. A 50% decrease in the level of disagreeable smell can be seen after using a 0.1% solution, while at 1 and 5% TCAS-CATA solution, the disagreeable odor indication falls to an hour. Figure 4B shows how odor reduction goes together with nitrite and nitrate release from slurries, showing that values over 0.1% can significantly reduce the odor while also releasing nitrogenated molecules. This data suggests that industrial slurries can be catalyzed without odor to generate compounds useful for farming.
Effect on odor and relationship with nutrients. Fig. A shows graph of progress over time (hr) of smell perception with mud in the presence and absence of increasing concentrations of TCAS-CATA (0.01 to 5%). In B, points with increasing concentrations of TCAS-CATA are shown, observing odor perception and nitrogen compound generation. The points are representative of the average ± SEM, from 3 independent experiments. The asterisk represents a p < 0.05 (ANOVA)
3.5 Catalyzed material and its effect on plant germination and development
The data suggest that industrial slurries catalyzed with TCAS-CATA release nitrogenated compounds without generating odors, which could be used in farming to add nutrients to the soil. To investigate this, studies were done on seed germination and plant growth using slaughterhouse slurries catalyzed with TCAS-CATA. Figure 5A shows seed development time after exposure to various industrial slaughterhouse slurries. All show a time reduction of almost 50% where the seed managed to show signs of development with seed opening. Considering this, Fig. 5B shows the stalk growth of Solanum lycopersicum exposed to slaughterhouse slurries catalyzed with increasing TCAS-CATA concentrations, where length over time increases after using slurry catalyzed with 1% or 5% TCAS-CATA. This data suggests that industrial slurries can be useful to stimulate plant growth in farms.
Germination time. Fig. A shows a graph of organic matter bar treated in absence or presence of TCAS 1% for 60 min. Hatching date and observation of the radicle were recorded. B shows a graph of progress over time of the stem of Solanum lycopersicum exposed to organic material with TCAS-CATA (0.1 to 5%). The bars and points are representative of the average ± SEM, of 6 independent experiments. The asterisk represents a p < 0.05 (ANOVA)
4 Discussion
The results of this study present the novel option of treating organic material from industrial slurries intended for special landfills with a catalyst, thus generating a fertilizer which provides increased organic material concentration in the soil for farming. The results are important since they reduce organic waste, are generated in a short time without odor, and provide rich nutrients and organic compounds to use in the soil. Our results suggested an enzymatic reaction-type mechanism. The different compounds present in TCAS-CATA mediated a Michaelis-Menten curve over the molecular degradation of the slurry and generation of biomolecules for soils. The two main decomposition models are anaerobic or fermentative, and aerobic. In the former, organic compounds decompose by the action of living organisms which do not require air in the normal sense. These organisms use nitrogen, phosphorus, and other nutrients to live and develop cellular protoplasm but reduce the organic nitrogen to organic acids and ammonia [17]. Other factors must also be considered for efficient decomposition, including temperature. This is a key factor since the decomposing biomass reaches its own internal temperature while the ambient temperature regulates the speed of the process [17, 18]. Other relevant factors are oxygen access and mechanical action resulting from currents which allow for the movement of layers of saprophytic agents, and the size of the particles or decomposition matrix [19]. Composting generally allows for creating optimal decomposition process conditions, namely a closed system which can generate the physiochemical conditions for this to occur, accumulating temperature and nutrients for the next phase in a much more efficient way [20]. In the first stage the compost reaches a high temperature and provides enough energy elements to begin the decomposition process. This stage is key because of the number of gasses generated, which should be kept sealed within the composting unit [21, 22]. This is one key problem in home composting, and the reason for the recommendation to not use certain initial products such as animal proteins, due to the residues formed and how the fermentation attracts vectors, especially fruit flies [10]. Along with the others which have begun to be described, such as the use of gasses and resistance transfer, there is the degradation of antibiotics [23, 24]. Various modifications have been indicated for composting systems, helping to reduce their impacts, using some substrate types to reduce gas generation [25] and also including improvements for compost production such as organic fertilizer (mulch) [13], the use of minerals [26] and other strategies. These factors indicate that we are still learning what happens inside a compost bin and how it impacts nutrient generation, especially nitrogen [27]. Doubtlessly, despite these problems, composting organic material is a good waste management option, but presents some disadvantages [28]. The data provide evidence that organic material can be quickly catalyzed and used as organic fertilizer, unlike what has been reported with composting [12], for farming purposes. In this case industrial slurries from various sources were used. In only hours, as seen in Fig. 1, they were able to generate a fertilizing solution. The compounds liberated by the TCAS-CATA solution can also vary by industrial slurry source, as seen in Fig. 2, offering the opportunity to mix various sources to fulfill specific farm requirements. For instance, the presence of calcium can be increased, as seen in Fig. 2A, when using wastes from marine industrial processes. Interestingly, catalyzation was independent of temperature, as observed in Fig. 3, allowing us to indicate that the action of generating nitrogenated compounds is more efficient and does not require special physiochemical conditions (Fig. 3). This can suggest a mechanism independent of fermentative processes. Along these lines, the problem of using organic materials is the generation of volatile acids (ammonia, sulfuric acid and others) which create repulsive odors [29] resulting from the action of anaerobic bacteria which begin the decomposition process [9]. This process can be reduced in duration with composting, because it is done via oxygen entry by mixture, but without any notable odor elimination, apart from the fact that certain protein sources are not recommended due to attracting vectors to the compost bin [12]. In this line, as observed in Fig. 4A, odor perception decreases with TCAS-CATA use, showing that hours after using it on industrial slurries and depending on the concentration, no unpleasant odors are perceived and oxygen injection is unnecessary, with only the compound having to be applied. This can also be correlated with the release of nitrogenated compounds, as seen in Fig. 4B, where TCAS-CATA concentrations over 0.1% significantly reduce odor and increase nitrogenated compound concentration in the solution. It is interesting to suggest the use of this material as organic compost, while in Chile regulations emphasize odor generation from wastes used for this purpose, along with a nitrogen source concentration allowing them to be used for certain farming purposes (Law # 20412/2010). This law indicates that there are various types of organic fertilizer, which can be classified as organic-animal origin, treated slurries and industrial or productive activities. The sources of these fertilizers are guanos (fresh and semi-composted), stabilized and fossilized guano, compost, humus, green fertilizer, crop wastes, wood and forestry industry wastes, agribusiness or municipal slurries or combinations of the preceding. Industrial slurries can therefore be used, but the odor they generate and their low nutrient availability make them unattractive for this use. As our results show, the system indicates that it is a feasible catalyst, reducing odor and leaving organic material available for plants. New studies to observe the effect in other wastes are needed, as well as to establish the concentration-response relation and kinetics to present a working protocol for these wastes.
Finally, Fig. 5 shows that treated wastes have potential use as fertilizer by reducing opening time for some seeds and increasing growth speed. These observations must be correlated with the land, but they allow us to suggest the use of industrial slurries as organic material sources for farming, without the odor problems which can arise with other organic fertilizers. This aligns with the circular economy policies declared by various ministerial organisms [30] and relates to organic waste reduction according to the new circular economic path in Chile (https://economiacircular.mma.gob.cl/hoja-de-ruta/), which opens opportunities for the use of industrial process slurries which had been sent solely to sanitary landfills as organic material for farming. The option also arises to catalyze these wastes as organic fertilizer in a much shorter time than currently practiced.
This information allows us to indicate that composting still has much evidence which needs exploration, continues to be perfected and is a real waste management solution. Since there is still much to contribute, be improved and even be replaced by some processes, though, it also appears viable. The compound has seen initial exploration, undergone years of development, and had its effects observed in the field. Its description is supported with in vitro and in vivo studies, suggesting it as an alternative to obtain nutrients for farming. While not all the experimental evidence is presented, it is available and can be contrasted with the decomposition and composting process, showing how it can help develop a circular economy innovation in the composting and organic waste management process.
5 Conclusion
This result helps us suggest a new industrial slurry management pathway. The compound TCAS-CATA can be used to take slurry and release it directly to the soil to improve nutrition quality. This is because the slurry is an excellent compound for the soil, but it is not bioavailable, and the changes in the slurry are slow and cause noxious smells. This strategy of using TCAS-CATA as a chemical catalyst generated a bioavailable nitrogen compound present in the slurry while not generating odor in the process. Future studies are required to explore other slurries and organic wastes and reduce garbage generation, in example at the domestic level.
References
Rahman MH, Haque KMS, Khan MZH. A review on application of controlled released fertilizers influencing the sustainable agricultural production: a cleaner production process. Environ Technol Innov. 2021;23:101697. https://doi.org/10.1016/j.eti.2021.101697.
Pacheco-Ruíz I, Zertuche-González JA, Arroyo-Ortega E, Valenzuela-Espinoza E. Agricultural fertilizers as alternative culture media for biomass production of Chondracanthus squarrulosus (Rhodophyta, Gigartinales) under semi-controlled conditions. Aquaculture. 2004;240:201–9. https://doi.org/10.1016/j.aquaculture.2004.05.044.
Zhang MJ, Jia JQ, Lu H, Feng MC, Yang WD. Functional diversity of soil microbial communities in response to supplementing 50% of the mineral N fertilizer with organic fertilizer in an oat field, journal of integrative. Agriculture. 2021;20:2255–64. https://doi.org/10.1016/S2095-3119(20)63331-7.
Santos AF, Almeida PV, Alvarenga P, Gando-Ferreira LM, Quina MJ. From wastewater to fertilizer products: alternative paths to mitigate phosphorus demand in European countries. Chemosphere. 2021;284:131258. https://doi.org/10.1016/j.chemosphere.2021.131258.
Xie D, Gao M, Yang M, Xu M, Meng J, Wu C, et al. Composting–a solution of eliminating a nitrite-rich wastewater by reusing it as a moisture conditioning agent. Chemosphere. 2021;284:131365. https://doi.org/10.1016/j.chemosphere.2021.131365.
Gurusamy NN, Puffer N, de Jongh C, Rodriguez Gil C, Aspray TJ. Effect of initial moisture content and sample storage duration on compost stability using the ORG0020 dynamic respiration test. Waste Manag. 2021;125:215–9. https://doi.org/10.1016/j.wasman.2021.02.048.
Lett LA. Las amenazas globales, el reciclaje de residuos y el concepto de economía circular. Rev Argent Microbiol. 2014;46:1–2. https://doi.org/10.1016/S0325-7541(14)70039-2.
Pascual JA, García C, Hernandez T. Comparison of fresh and composted organic waste in their efficacy for the improvement of arid soil quality. Bioresour Technol. 1999;68:255–64. https://doi.org/10.1016/S0960-8524(98)00160-6.
Griffiths K, Krosch MN, Wright K. Variation in decomposition stages and carrion insect succession in a dry tropical climate and its effect on estimating postmortem interval. Forensic Sci Res. 2020:1–9. https://doi.org/10.1080/20961790.2020.1733830.
A. Selvam, X. Wang, J. Wong, Chapter Five - Food Waste Composting: Challenges and Possible Approaches, in: J. Wong, G. Kaur, M. Taherzadeh, A. Pandey, K. Lasaridi (Eds.) Current Developments in Biotechnology and Bioengineering, Elsevier2021, pp. 137–162.
Estrella-González MJ, Jurado MM, Suárez-Estrella F, López MJ, López-González JA, Siles-Castellano A, et al. Enzymatic profiles associated with the evolution of the lignocellulosic fraction during industrial-scale composting of anthropogenic waste: comparative analysis. J Environ Manag. 2019;248:109312. https://doi.org/10.1016/j.jenvman.2019.109312.
Bao Y, Feng Y, Qiu C, Zhang J, Wang Y, Lin X. Organic matter- and temperature-driven deterministic assembly processes govern bacterial community composition and functionality during manure composting. Waste Manag. 2021;131:31–40. https://doi.org/10.1016/j.wasman.2021.05.033.
Stewart-Wade SM. Efficacy of organic amendments used in containerized plant production: part 1 – compost-based amendments. Sci Hortic. 2020;266:108856. https://doi.org/10.1016/j.scienta.2019.108856.
Abdalla MA, Endo T, Maegawa T, Mamedov A, Yamanaka N. Effectiveness of organic amendment and application thickness on properties of a sandy soil and sand stabilization. J Arid Environ. 2020;183:104273. https://doi.org/10.1016/j.jaridenv.2020.104273.
Schueler TA, Dourado ML, Videira SS, da Cunha CD, Rizzo ACL. Biosolubilization of verdete: an alternative potassium source for agriculture fertilizer. Biocatalysis Agri Biotechnol. 2021;34:102031. https://doi.org/10.1016/j.bcab.2021.102031.
Xu Z, Ma Y, Zhang L, Han Y, Yuan J, Li G, et al. Relating bacterial dynamics and functions to gaseous emissions during composting of kitchen and garden wastes. Sci Total Environ. 2021;767:144210. https://doi.org/10.1016/j.scitotenv.2020.144210.
Boros G, Czeglédi I, Erős T, Preiszner B. Scavenger-driven fish carcass decomposition and phosphorus recycling: laboratory experiments with freshwater fish and crayfish. Freshwater Biology n/a. 2020. https://doi.org/10.1111/fwb.13576.
Benbow ME, Receveur JP, Lamberti GA. Death and decomposition in aquatic ecosystems. Front Ecol Evol. 2020;8. https://doi.org/10.3389/fevo.2020.00017.
Y.-X. Guo, Q.-J. Chen, Y. Qin, Y.-R. Yang, Q.-Z. Yang, Y.-X. Wang, Z.-a. Cheng, N. Cao, G.-Q. Zhang, Succession of the microbial communities and function prediction during short-term peach sawdust-based composting, Bioresour Technol 332 (2021) 125079. https://doi.org/10.1016/j.biortech.2021.125079.
Canet R, Pomares F. Changes in physical, chemical and physico-chemical parameters during the composting of municipal solid wastes in two plants in Valencia. Bioresour Technol. 1995;51:259–64. https://doi.org/10.1016/0960-8524(94)00132-K.
Andreux F. Chapter 2 - humus in world soils. In: Piccolo A, editor. Humic substances in terrestrial ecosystems. Amsterdam: Elsevier Science B.V; 1996. p. 45–100.
Graça J, Murphy B, Pentlavalli P, Allen CCR, Bird E, Gaffney M, et al. Bacterium consortium drives compost stability and degradation of organic contaminants in in-vessel composting process of the mechanically separated organic fraction of municipal solid waste (MS-OFMSW). Bioresource Technol Rep. 2021;13:100621. https://doi.org/10.1016/j.biteb.2020.100621.
Ezugworie FN, Igbokwe VC, Onwosi CO. Proliferation of antibiotic-resistant microorganisms and associated genes during composting: an overview of the potential impacts on public health, management and future. Sci Total Environ. 2021;784:147191. https://doi.org/10.1016/j.scitotenv.2021.147191.
He X, Han L, Huang G. Analysis of regulative variables on greenhouse gas emissions and spatial pore gas concentrations with modeling during large-scale trough composting. J Clean Prod. 2020;277:124066. https://doi.org/10.1016/j.jclepro.2020.124066.
Wong JWC, Fung SO, Selvam A. Coal fly ash and lime addition enhances the rate and efficiency of decomposition of food waste during composting. Bioresour Technol. 2009;100:3324–31. https://doi.org/10.1016/j.biortech.2009.01.063.
Margaritis M, Psarras K, Panaretou V, Thanos AG, Malamis D, Sotiropoulos A. Improvement of home composting process of food waste using different minerals. Waste Manag. 2018;73:87–100. https://doi.org/10.1016/j.wasman.2017.12.009.
Wang X, Selvam A, Chan M, Wong JWC. Nitrogen conservation and acidity control during food wastes composting through struvite formation. Bioresour Technol. 2013;147:17–22. https://doi.org/10.1016/j.biortech.2013.07.060.
Al-Ghouti MA, Khan M, Nasser MS, Al-Saad K, Heng OE. Recent advances and applications of municipal solid wastes bottom and fly ashes: insights into sustainable management and conservation of resources. Environ Technol Innov. 2021;21:101267. https://doi.org/10.1016/j.eti.2020.101267.
Zang B, Li S, Michel FC, Li G, Zhang D, Li Y. Control of dimethyl sulfide and dimethyl disulfide odors during pig manure composting using nitrogen amendment. Bioresour Technol. 2017;224:419–27. https://doi.org/10.1016/j.biortech.2016.11.023.
González JM, Ovalle MJ, Salazar M. La economía circular como respuesta alternativa a los desafíos de la alimentación: análisis de caso para la situación de Chile, Revista Chilena de. Relac Int. 2018;2:94–104.
Acknowledgments
We would like to thank Laboratorio Tonalli Ltda., for measurements and interpretations and we are in debt with Scott Stiefel for MS edition.
Author information
Authors and Affiliations
Contributions
The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Plaza, T., Scott, I., Vega, I. et al. Management of industrial slurries with a chemical catalyst: generation of organic sustainable solution. GRN TECH RES SUSTAIN 2, 6 (2022). https://doi.org/10.1007/s44173-022-00006-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44173-022-00006-y