Abstract
Diabetic mellitus is a chronic, incurable metabolism disorder caused by the lack of secretion of insulin by the pancreas. Currently, several plants are used for the treatment of diabetic mellitus. Cichorium intybus (C. intybus) is one of the medicinal plants used traditionally by Asian people to treat diabetics. In this regard, the aim of the work is to prepare different selected plant crude extracts and determine them for in vivo diabetic activity against streptozotocin-induced diabetic rats. At first, the plant leaves powder was defatted with petroleum spirit. Then the defatted powder was extracted by water with a ratio of 1:8 for 48 h using the maceration method. The water was evaporated by using a rotary evaporator. The aqueous extract of the selected plant was administered orally (100 mg/kg, 250 mg/kg, and 500 mg/kg) at intervals of 0, 2, 4 and 6 h compared to glibenclamide (3 mg/kg) and to measure blood glucose level by using a glucometer. The results showed that the blood glucose level decreased gradually with the increase of the time intervals. In addition, the blood glucose decreasing rate was increased with the applied dose increased. In conclusion, the selected plant water crude extract could be used to decrease the blood glucose level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Diabetes mellitus is a long-lasting metabolic disease caused by a lack of insulin secretion from the pancreas. It is characterized by high blood sugar after food and or an empty state. In its severe form, it is accompanied by protein wasting and ketosis [1]. Genetic factors and lifestyle factors, such as increased fat intake and reduced exercise are the leading causes of diabetes [2]. National Diabetics Statistics Report (NDSR) showed that a total of 37.3 million people have diabetes in the USA, which is about 11.3% of the population. It includes 28.7 million diagnosed people and 8.6 million undiagnosed people. This report also showed that about 96 million US people above 18 years old have prediabetics.
According to an estimate from the International Diabetes Federation (IDF), there would be 69.9 million diabetic patients in India by 2025. Nowadays, diabetes affects up to 11% of India’s urban and 3% of its rural populations over the age of 15. The availability of statistical information on people with diabetes is almost universal. Diabetes is a chronic condition that can lead to a number of other illnesses. It encompasses numerous conditions such as liver disease, kidney disease, heart disease, heart failure, and brain stroke. Due to diabetes and diseases brought on by diabetes, there is an extremely high death rate [3].
The literature search showed that several medicinal plants are traditionally used to treat diabetes and its related diseases. Plants and their formulated medicines are used widely in alternative and complementary medicine systems. Plant-based medicines are safe, active, and widely available. Therefore, more than 80% of people have used traditional medicine to treat various diseases reported by the WHO. The demand for plant-based medicines is increasing tremendously due to their safety and availability. However, there needs to be more data available on the medicinal plants that are used traditionally for the treatment of diseases. There has been an increasing demand to evaluate the antidiabetic properties of direct plant extracts rather than synthetic ones (insulin, sulphonylureas, biguanides, and thiazolidinediones) due to their toxic effects [4].
A medicinal plant called Cichorium intybus (C. intybus) has been used in the past to cure a number of ailments, including liver, diuretic, cardiac, hepatomegaly, anorexia, dyspepsia, jaundice, and others [5, 6]. In the present study, the acute effects of oral administration of a water-based crude extract of C. intybus on glucose levels were investigated in normal, normal hyperglycemic, and diabetic rats. C. intybus L. is a member of the Asteraceae family. Known locally as kasni or chicory in India, it is a conventional herbal treatment that the Prophet Muhammad (SWT) first used 1400 years ago. [7]. Numerous common names for the chosen plant are used throughout the world, including coffee weed, blue sailors, cornflower, wild chicory, blue daisy, garden chicory, blue weed, bunk, and many more [8, 9]. This plant species can grow anywhere between 20 and 150 cm tall. The plant has long, thick brown roots and green stems. Its leaves are 2 to 5 inches long. The seeds of the chosen plant are extremely potent and are used in herbal medicine systems to treat a variety of liver conditions [7]. The main constituents of Chicory reported are coumarins, amino acids, flavonoids, polysaccharides, sterols, glycosides, sesquiterpenes, lacton and triterpenoids [10,11,12,13]. The seeds also contain various minerals, especially potassium, magnesium, phosphorus, calcium, sodium, iron, zinc, manganese, copper and others [14, 15]. The literature also showed that the chicory has reported antihepatotoxic, anti-inflammatory, antimicrobial, antioxidant, anticoagulant, anticancer and antimalarial activity [10,11,12,13,14,15]. Rats were used to test the hepatoprotective effects of aqueous extracts from the seeds of the chosen plant. When the aqueous extract was tested against carbon tetrachloride (CCl4) and paracetamol-induced toxicity, it was discovered to be hepatoprotective at various concentrations [6]. The work’s objective is to make various aqueous extracts of chosen plants and assess their in vivo antidiabetic activity against streptozotocin-induced diabetic rats.
2 Experiment
2.1 Chemicals and reagents
The plant material was purchased locally at Khari Baoli in Delhi. The taxonomist from Jamia Hamdard, Department of Botany recognized and verified plant samples using voucher specimens (No 40). The sample was delivered to Jamia Hamdard’s Faculty of Pharmacy’s Phytochemistry Research Laboratory in New Delhi. All of the analytical-grade chemicals and reagents were bought from Mercodia AB in Uppsala, Sweden, and Merck from Germany.
2.2 Extracts preparation
The selected plant samples were dried at room temperature for three days under the sun. Ball mills were used to grind the samples into a coarse powder. The powder samples (250 g) were defatted with petroleum spirit (1000 mL), extracted with distilled water (1:8; 500 mL), and stirred for 3–4 h with a magnetic stirrer at temperatures between 70 and 80 °C. A rotary evaporator was used to filter and concentrate the extract. When the extracts were dissolved in distilled water, the residues (24.65 g) were retained for testing for antidiabetic activity. Three concentrations were created using the distilled water: 100 mg/kg, 250 mg/kg, and 500 mg/kg.
2.3 Animals
Male Wistar rats weighing 150–200 g were purchased from Jamia Hamdard University’s Central Animal Home. The rats were 6 to 8 weeks old on average. Groups of animals were kept in PVC cages with food and water. The animals went without food or water for 12 h before treatment. The standard drugs and all prepared dose extracts were administered orally.
2.4 Induction of diabetes
Streptozotocin (STZ) (65 mg/kg) was injected intraperitoneally once to cause diabetes in rats, which was freshly produced in citrate buffer with a pH value of 4.5. After administering STZ for 48 h, diabetes was identified by using a glucometer to measure blood glucose levels. The following are the results of a survey conducted by the American Psychological Association. Animals with diabetes were divided into groups at random. The diabetic rats were administered a 5% glucose solution orally to prevent hypoglycemia for the first 24 h after the STZ injection. Rats were starved for 16 h before receiving STZ [16].
2.5 Effect of plant extracts on diabetic rats
Rats of Group I was normal control [15]. Group II rats were diabetic control. Groups I and II each received a vehicle for the duration of the study. Group III received the reference standard, glibenclamide (3 mg/kg), as compared to Group IV, which received C. intybus extract at doses of 100, 250, and 500 mg/kg. The control group received only the buffer solution. Blood was obtained, and the blood glucose level was assessed immediately prior to, 2, 4, and 6 h after the administration of the plant drug dose.
2.6 Effect of 21 days administration of plant extracts on diabetic rats
Only the buffer was given to the control rats. Rats in Group I were healthy and behaved as the healthy control. Group II was used to control diabetes. Rats in Groups I and II received a vehicle during the tests, while Group III received an intraperitoneal injection of insulin (5 U/kg). Group IV received 500 mg/kg every day for 21 days of plant extracts of C. intybus. The dosage was given each day at 10:00 a.m. daily. Initial, 10th, and 21st-day non-fasting blood glucose levels were determined before administering the plant drug.
Animal body weight was carefully tracked so that the dose could be adjusted. Using disposable syringes, blood was drawn from each animal’s tail vein on the 22nd day after they had fasted all night. Centrifugation was used to separate the blood serum from the blood for 20 min at 3000 rpm. Using commercially available kits, the estimation of total cholesterol, triglycerides, low-density lipoprotein (LDL), and low-density lipoprotein (HDL) was done. Using an enzyme-linked immunosorbent assay (ELISA) kit, the serum insulin was calculated.
2.7 Biochemical estimations
2.7.1 Blood glucose
To determine the level of blood glucose, Glucometer and diabetic test strips One Touch Basic Plus (Lifescan Inc. California, USA) were used (glucose oxidase method).
2.7.2 Insulin ELISA
The rat Insulin ELISA kit was used to determine the serum insulin levels (Mercodia AB, Uppsala, Sweden). In order to determine the insulin level, we strictly adhered to the procedure and kit supplied by the manufacturer. Two monoclonal antibodies are directed toward various antigenic features on the insulin molecule using the direct sandwich approach. For measuring the concentration of unidentified sample a calibration curve was created by plotting the absorbance values for the standard solutions against the concentration of insulin.
2.7.3 Lipid profile
Using commercially available kit, the total blood cholesterol (TC), triglycerides (TG), and HDL were calculated (Span Diagnostics, India). The procedure provided by the kit’s manufacturer was used. The following formulas were used to determine the LDL and very low density lipoprotein (VLDL) levels. [16, 17].
2.8 Statistical analysis
Mean standard error of the mean is the unit of measurement for values. A one-way analysis of variance and Dunnett’s ‘t’ test were used to determine statistical significance. When the P-value was less than 0.05, the differences between the values were deemed to be significant.
3 Results
Since old times, plants have been used to cure various diseases. Plants contain different classes of organic and inorganic chemical compounds significantly responsible for curing diseases. Many biologically active medicinal plants are available in Oman. They are traditionally used in different ethnic communities to treat various ailments because of the therapeutically active phytochemicals present in the plants. Currently, one of the most severe metabolic disorders is diabetes. Various kinds of medications are available in the local and international markets to improve or cure the symptoms of diabetes. However, these available drugs for managing diabetes are expensive, and they have several side effects. In this situation, plant-based medicines are significantly important in managing diabetes as they are cost-effective and have fewer side effects. One of the active plants traditionally used in India to treat people with diabetes is C. intybus; however, there is limited information regarding phytochemicals and biological activities available about the selected plant species. Therefore, the aim of the work is to prepare different selected plant crude extracts and determine them for in vivo antidiabetic activity against streptozotocin-induced diabetic rats.
3.1 Effect of plant extracts on diabetic rats
Streptozotocin administration after 48 h (65 mg/kg) dramatically raised blood sugar levels (Table 1). At 500 mg/kg, C. intybus extract significantly reduced blood sugar, and the effect persisted for 6 h (P 0.01). At 250 mg/kg, extracts were only marginally active, while a dose of 100 mg/kg had a short-term, significant effect on blood sugar levels.
3.2 Effect of a 21-day plant extract administration on the blood sugar levels of diabetic rats
On days 10 and 21, a C. intybus extract (500 mg/kg) significantly reduced blood glucose levels. The percentage drop demonstrated that the decrease was significantly greater than the acute therapy (Table 2).
3.3 Effect of a 21-day plant extract administration on serum lipid profile of diabetic rats
The varied serum lipid profiles were increased after streptozotocin administration. In comparison to diabetes management, insulin therapy considerably reduced the lipid profile, including total cholesterol, LDL, HDL, VLDL, and triglycerides. Moreover, C. intybus water extract significantly reduced the lipid profile (P0.01) and was also observed to reduce the atherogenic index (Table 3).
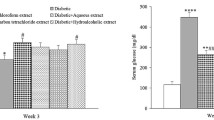
3.4 Effect of a 21-day plant extract administration on serum insulin levels of diabetic rats
The administration of C. intybus extract significantly decreased the serum insulin levels in the diabetic-induced mice. The serum insulin levels were not considerably raised by the 500 mg/kg dose, though (Table 4).
3.5 Effect of a 21-day plant extract administration on liver and body weight of diabetic rats
Rats that were given streptozotocin to induce diabetes had significantly lower body weights than animals in the control group. Yet, the treatment of insulin improved the body weight loss as compared to the diabetes control group. Also, a dose of 500 mg/kg of C. intybus extract did not significantly inhibit weight loss as compared to the diabetic control group (Table 5).
4 Discussion
At present, diabetes is a common pancreas disorder that affects at least 100 million people globally. This number will double by the year 2030. In most of the lowest-income countries, including India, Bangladesh, and Pakistan, there has been an alarming increase in the number of diabetics over the past decade. Several pharmaceutical drugs, such as thiazolidinediones, biguanides, and insulin, are used in modern medicine to control blood glucose. These drugs have hypoglycemic activities but can produce several health problems, such as neural disorders, diarrhea, heart diseases, and many others. All these health problems have been managed by using alternative herbal drugs. They have lesser side effects and, however, improved therapeutic values. The present study has evaluated the antidiabetic properties of the aqueous extract of C. intybus.
Beta-cells in the pancreatic islets of Langerhans die after being injected with streptozotocin (STZ) [16]. The loss of -cells in the pancreas causes a considerable reduction in serum insulin levels [17]. The drug’s modest impact on STZ-induced diabetic rats can be related to the fact that there are practically any -cells in the pancreas to create any secretagogue activity. It has already been documented that this animal took glibenclamide [18]. Water extracts of the test drug sample, C. intybus, only displayed a moderately potent antihyperglycaemic effect in rats with STZ-induced diabetes.
The extracts’ potential mechanism could be an increase in peripheral glucose uptake or a reduction in liver endogenous glucose synthesis. At this point, it is impossible to rule out the potential of one or more of these pathways, with each extract contributing to the activity. The process involving intestinal delay or glucose inhibition can be disregarded because the study’s subjects’ animals fasted the night before the experiment began.
In a chronic STZ-induced diabetes investigation, an aqueous extract of every plant was employed at a concentration of 500 mg/kg based on the findings in the acute models. The lack of serum insulin in STZ-diabetic rats have been extensively established [19]. In the current investigation, diabetic control rats’ blood insulin levels were significantly lower than those of normal rats. In STZ-induced diabetes rats, endogenous insulin has a very small function.
The results demonstrate that chronic C. intybus extract therapy has a significant antihyperglycaemic effect in diabetic rats caused by STZ. The form of diabetes that STZ produces in rats is IDDM. The extracts’ extra-pancreatic mode of action is further supported by the fact that they were able to lower blood sugar levels in these animals when compared to untreated, healthy rats [20].
In the current study, it was found that the serum insulin levels of diabetic control rats were significantly lower than those of normal control rats (Table 4). The extract of the test drug did not significantly increase the levels of insulin after treatment. It means that the extracts did not cause any pancreatic cell regrowth. In order to achieve the antihyperglycemic effect, they did not boost insulin secretion. If the extracts had any insulinotropic activity at all, it would have only been in a very small portion of the accessible -cell; yet, this does not rule out the possibility.
Increased lipid levels are associated with diabetes illnesses, along with hyperglycemia [21, 22]. Plasma triglyceride and cholesterol levels are higher in patients with various types of diabetes than in healthy individuals [23]. Early atherosclerosis appears to be significantly influenced by the lipid profile, which is altered in the serum of diabetic individuals [24] and includes an increase in total cholesterol levels and triglyceride.
Insulin normally causes the lipoprotein lipase enzyme to become active and hydrolyze triglycerides. Lack of insulin prevents the enzymes from being activated, leading to hypertriglyceridemia. Insulin-dependent diabetes has a greater plasma free fatty acid level as a result of improved free fatty acid release from fat depots [23].
In diabetes studies, streptozotocin treatment results in an insulin shortage. Triglycerides, LDL, and VLDL levels rising are symptoms of hyperglycemia and dyslipidemia [25]. The liver and some other tissues participate in a variety of metabolic processes, including oxidation, uptake and metabolic conversion of free fatty acids, the production of phospholipids and cholesterol, and the secretion of specific kinds of plasma lipoproteins.
Lowering blood cholesterol levels with dietary changes or drug therapy seems to be linked to a decreased risk of vascular disease and its consequences. Many plants and herbal remedies have been shown to reduce blood sugar and cholesterol levels [26,27,28].
5 Conclusion
According to the findings, streptozotocin-induced diabetes in rats resulted in higher levels of circulating lipids. When compared to normal untreated control rats, it was discovered that the serum cholesterol levels and several lipoprotein fractions, including LDL, HDL, VLDL, and triglycerides, had increased. It was discovered that STZ-diabetic rats had greater atherogenic indices than normal rats. LDL and VLDL to HDL ratio is represented by the atherogenic index. Atherogenesis is more likely to occur at higher values, which increases the risk of cardiovascular problems. Insulin therapy reduced the amount of elevated lipoproteins and total cholesterol. When C. intybus extract was administered, the amount of circulating lipids was reduced. Moreover, they reduced the atherogenic index. The increase in HDL was greater and the atherogenic index was reduced more successfully by C. intybus. The findings clearly show that C. intybus aqueous extracts had antidiabetic action. To determine the mechanism of the extract’s antidiabetic effect, more research is needed.
Data availability
The data sets created as part of the current study are available.
References
Bell G (1991) Molecular defects in diabetes mellitus. Diabetes 40:413–417
Lipman RL, Raskin P, Love T, Triebasser J, Lecocq FR, Schnure JJ (1972) Glucose intolerance during decreased physical activity in man. Diabetes 21:101–107
Hazra B, Sarkar R, Bhattacharyya S, Roy P (2002) Tumour inhibitory activity of chicory root extract against Ehrlich ascites carcinoma in mice. Fitoterapia 73:730–733
Valiathan MS (1998) Healing plants. Curr Sci 75:1122–1126
Ahmed B, Al-Howiriny TA, Siddiqui AB (2003) Antihepatotoxic activity of seeds of Cichorium intybus. J Ethnopharmacol 87:237–240
Gadgoli CH, Mishra SH (1994) Antihepatotoxic activity of Cichorium intybus. J Ethanopharmacol 56(4):157–160
Saroja S, Padma PR, Radha P, Thilagavathy P (2000) Enzymic and nonenzymic antioxidants in Cichorium intybus. J Med Aromat Plant 22:37–42
Bais HP, Ravishankar GA (2001) Cichorium intybus L. cultivation, processing, utility, value addition and biotechnology, with an emphasis on current status and future prospects. J Sci Food Agri 81(5):467–484
Janda K, Gutowska I, Geszke-Moritz M, Jakubczyk K (2021) The common cichory (Cichorium intybus L.) as a source of extracts with health-promoting properties—a review. Mole 26:1814–1820
Balbaa SI, Zaki AY, Abdel-Wahab SM, Denshary ASMEI, Motazz-Bellah, (1973) Preliminary Phytochemical and pharmacological investigations of the roots of different varieties of Cichorium intybus. Planta Med 24:134–144
Lakany-El AM, Aboul-Ela A-G, Mohamed M, Mekkey H (2004) Chemical constituents and biological activities of Cichorium intybus L. Nat Prod Sci 10(2):69–73
Norbaek R, Nielsen K, Kondo T (2002) Anthocyanin from flowers of Cichorium intybus. Phytochem 60:357–369
Seto M, Miyase T, Umehara K, Hirano Y, Otani N (1988) Sesquiterpene lactone from Cichorium endiva L., C. intybus L. and Cytotoxic activity. Chem Pharma Bull 36(7):2573–2429
Bischoff TA, Kelley CJ, Karchesy Y, Laurantos M, Nguyen-Dinh P, Ghafoor Arefim A (2004) Antimalarial activity of Lactucin and Lactucopicrin: sesquiterpene lactones isolated from Cichorium intybus L. J Ethnopharmacol 95:455–457
Jayasooriya AP, Sakono M, Kawano YC, M, Yamamoto K, Fukuda N, (2000) Effects of Momordica charantia powder on serum glucose levels and various lipid parameters in rats fed with cholesterol-free and cholesterol-enriched diets. J Ethnopharmacol 72:331–336
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of low-density lipoprotein cholesterol in plasma, without use of the preparative centrifuge. Clin Chem 18:499
Mukherjee B (2003) Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative disease. J Ethanopharmacol 84:131–138
Wealth of India (1992). Publication and Information Directorate, Council of Scientific and Industrial Research, New Delhi, 3: 555
Wohaieb SA, Godin DV (1987) Alterations in free radical tissue-defense mechanisms in streptozotocin-induced diabetes in rats. Diabetes 36:1014–1018
Brown GB, Xue-Qiao Sacco DE, Alberts JJ (1993) Lipid lowering and plaque regression. New insights into prevention of plaque disruption and clinical events in coronary disease. Circulation 87:1781–1791
Jay RH, Betteridge DJ (1991) In Textbook of Diabetes, Edited by Pickup J, Williams G, Blackwell Scientific Publications, Oxford, 701.
Chase PH, Glasgow AM (1976) Juvenile diabetes and serum lipids and lipoproteins. Am J Dis Child 130:1113
Betteridge DJ (1994) Diabetic dyslipidemia. Am J Med Suppl 6A(96):25S-31S
Brown MS, Goldstein JL (1994) Harrison’s Principles of Internal Medicine, Edited by Isselbacher K.J., Braunwald E. McGraw Hill Inc, International Edition. 2068.
Elsner M, Guldbakke B, Tiedge M, Munday R, Lenzen S (2000) Relative Importance of transport and alkylation for pancreatic beta-cell toxicity of streptozotocin. Diabetologia 43:1528–1533
Gilman AG, Rall TW, Nies AS, Tayer P (2001) Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 10th edn. McGraw-Hill, New Delhi, India, pp 1317–1322
Graham ML, Janecek JL, Kittredge JA, Hering BJ, Schuurman H (2011) The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources. Comp Med 61(4):356–360
Vhora N, Naskar U, Hiray A, Kate AS, Jain A (2020) Recent advances in in-vitro assays for type 2 diabetes mellitus: an overview. Rev Diabet Stud 16(1):13–23
Acknowledgements
We are thankful to Jamia Hamdard and the University of Nizwa for providing the necessary facilities to complete this research work and publish this manuscript.
Funding
We didn’t receive any funding for this study.
Author information
Authors and Affiliations
Contributions
MSA: Data collection and data analysis. MR: Samples collection. MAH: Manuscript edit and process. MA: Date analysis.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akhtar, M.S., Rafiullah, M., Hossain, M.A. et al. Antidiabetic activity of Cichorium intybus L water extract against streptozotocin-induced diabetic rats. J.Umm Al-Qura Univ. Appll. Sci. 9, 565–571 (2023). https://doi.org/10.1007/s43994-023-00066-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43994-023-00066-1