Abstract
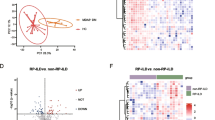
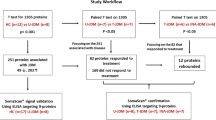
Dermatomyositis (DM) is a heterogeneous autoimmune disease associated with numerous myositis specific antibodies (MSAs) in which DM with anti-melanoma differentiation-associated gene 5-positive (MDA5 + DM) is a unique subtype of DM with higher risk of developing varying degrees of Interstitial lung disease (ILD). Glycosylation is a complex posttranslational modification of proteins associated with many autoimmune diseases. However, the association of total plasma N-glycome (TPNG) and DM, especially MDA5 + DM, is still unknown. TPNG of 94 DM patients and 168 controls were analyzed by mass spectrometry with in-house reliable quantitative method called Bionic Glycome method. Logistic regression with age and sex adjusted was used to reveal the aberrant glycosylation of DM and the association of TPNG and MDA5 + DM with or without rapidly progressive ILD (RPILD). The elastic net model was used to evaluate performance of glycans in distinguishing RPLID from non-RPILD, and survival analysis was analyzed with N-glycoslyation score by Kaplan–Meier survival analysis. It was found that the plasma protein N-glycome in DM showed higher fucosylation and bisection, lower sialylation (α2,3- not α2,6-linked) and galactosylation than controls. In MDA5 + DM, more severe disease condition was associated with decreased sialylation (specifically α2,3-sialylation with fucosylation) while accompanying elevated H6N5S3 and H5N4FSx, decreased galactosylation and increased fucosylation and the complexity of N-glycans. Moreover, glycosylation traits have better discrimination ability to distinguish RPILD from non-RPILD with AUC 0.922 than clinical features and is MDA5-independent. Survival advantage accrued to MDA5 + DM with lower N-glycosylation score (p = 3e-04). Our study reveals the aberrant glycosylation of DM for the first time and indicated that glycosylation is associated with disease severity caused by ILD in MDA5 + DM, which might be considered as the potential biomarker for early diagnosis of RPILD and survival evaluation of MDA5 + DM.




Similar content being viewed by others
Data Availability
The main data supporting the findings of this study are available within the manuscript and its Supplementary materials. All data generated, including both raw images and analyzed datasets for the figures in this study, are available upon request from the corresponding author.
Abbreviations
- DM:
-
Dermatomyositis
- MSAs:
-
Myositis specific antibodies
- MDA5 + DM:
-
DM with anti-melanoma differentiation-associated gene 5-positive
- ILD:
-
Interstitial lung disease
- TPNG:
-
Total plasma N-glycome
- RPILD:
-
Rapidly progressive interstitial lung disease
- SLE:
-
Systemic lupus erythematosus
- RA:
-
Rheumatoid arthritis
- IBD:
-
Inflammatory bowel disease
- MALDI-TOF-MS:
-
Matrix assisted laser desorption/ionization time of flight mass spectrometry
- ASS:
-
Anti-synthetase syndrome
- Anti-ARSs:
-
Anti-synthetases antibodies
- HRCT:
-
High-resolution computed tomography
- PaO2 :
-
Partial pressure of arterial oxygen
- SD:
-
Standard deviation
- ROC:
-
Receiver operating characteristic curves
- A:
-
Antennae
- S:
-
Sialylation
- F:
-
Fucosylation
- B:
-
Bisection
- G:
-
Galactosylation
- TM:
-
High-mannose glycan types
- THy:
-
Hybrid glycan types
- TC:
-
Complex glycan types
- AUC:
-
Area under curve
- SA:
-
Sialic acid
- HC:
-
Healthy controls
- NK:
-
Natural killer
References
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (second of two parts). N Engl J Med 292(8):403–407. https://doi.org/10.1056/nejm197502202920807
Cavagna L, Trallero-Araguás E, Meloni F, Cavazzana I, Rojas-Serrano J, Feist E, Zanframundo G, Morandi V, Meyer A, da PereiraSilva JA, Matos Costa CJ, Molberg O, Andersson H, Codullo V, Mosca M, Barsotti S, Neri R, Scirè C, Govoni M, Furini F, Lopez-Longo FJ, Martinez-Barrio J, Schneider U, Lorenz HM, Doria A, Ghirardello A, Ortego-Centeno N, Confalonieri M, Tomietto P, Pipitone N, Rodriguez Cambron AB, Blázquez Cañamero M, Voll RE, Wendel S, Scarpato S, Maurier F, Limonta M, Colombelli P, Giannini M, Geny B, Arrigoni E, Bravi E, Migliorini P, Mathieu A, Piga M, Drott U, Delbrueck C, Bauhammer J, Cagnotto G, Vancheri C, Sambataro G, De Langhe E, Sainaghi PP, Monti C, Gigli Berzolari F, Romano M, Bonella F, Specker C, Schwarting A, Villa Blanco I, Selmi C, Ceribelli A, Nuno L, Mera-Varela A, Perez Gomez N, Fusaro E, Parisi S, Sinigaglia L, Del Papa N, Benucci M, Cimmino MA, Riccieri V, Conti F, Sebastiani GD, Iuliano A, Emmi G, Cammelli D, Sebastiani M, Manfredi A, Bachiller-Corral J, Sifuentes Giraldo WA, Paolazzi G, Saketkoo LA, Giorgi R, Salaffi F, Cifrian J, Caporali R, Locatelli F, Marchioni E, Pesci A, Dei G, Pozzi MR, Claudia L, Distler J, Knitza J, Schett G, Iannone F, Fornaro M, Franceschini F, Quartuccio L, Gerli R, Bartoloni E, Bellando Randone S, Zampogna G, Gonzalez Perez MI, Mejia M, Vicente E, Triantafyllias K, Lopez-Mejias R, Matucci-Cerinic M, Selva-O’Callaghan A, Castañeda S, Montecucco C, Gonzalez-Gay MA (2019) Influence of antisynthetase antibodies specificities on antisynthetase syndrome clinical spectrum time course. J Clin Med. https://doi.org/10.3390/jcm8112013
Clerc F, Reiding KR, Jansen BC, Kammeijer GS, Bondt A, Wuhrer M (2016) Human plasma protein N-glycosylation. Glycoconj J 33(3):309–343. https://doi.org/10.1007/s10719-015-9626-2
Clerc F, Novokmet M, Dotz V, Reiding KR, de Haan N, Kammeijer GSM, Dalebout H, Bladergroen MR, Vukovic F, Rapp E, Targan SR, Barron G, Manetti N, Latiano A, McGovern DPB, Annese V, Lauc G, Wuhrer M (2018) Plasma N-glycan signatures are associated with features of inflammatory bowel diseases. Gastroenterology 155(3):829–843. https://doi.org/10.1053/j.gastro.2018.05.030
Cvetko A, Kifer D, Gornik O, Klarić L, Visser E, Lauc G, Wilson JF, Štambuk T (2020) Glycosylation alterations in multiple sclerosis show increased proinflammatory potential. Biomedicines 8(10):410. https://doi.org/10.3390/biomedicines8100410
DeWane ME, Waldman R, Lu J (2020) Dermatomyositis: clinical features and pathogenesis. J Am Acad Dermatol 82(2):267–281. https://doi.org/10.1016/j.jaad.2019.06.1309
Dotz V, Lemmers RFH, Reiding KR, Hipgrave Ederveen AL, Lieverse AG, Mulder MT, Sijbrands EJG, Wuhrer M, Van Hoek M (2018) Plasma protein N-glycan signatures of type 2 diabetes. Biochim Biophys Acta Gen Subj 1862(12):2613–2622. https://doi.org/10.1016/j.bbagen.2018.08.005
Ereño-Orbea J, Sicard T, Cui H, Mazhab-Jafari MT, Benlekbir S, Guarné A, Rubinstein JL, Julien JP (2017) Molecular basis of human CD22 function and therapeutic targeting. Nat Commun 8(1):764. https://doi.org/10.1038/s41467-017-00836-6
Friedman J, Hastie T, Tibshirani R (2010) Regularization paths for generalized linear models via coordinate descent. J Stat Softw 33(1):1–22
Johnson WE, Li C, Rabinovic A (2007) Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8(1):118–127. https://doi.org/10.1093/biostatistics/kxj037
Kurimoto A, Kitazume S, Kizuka Y, Nakajima K, Oka R, Fujinawa R, Korekane H, Yamaguchi Y, Wada Y, Taniguchi N (2014) The absence of core fucose up-regulates GnT-III and Wnt target genes: a possible mechanism for an adaptive response in terms of glycan function. J Biol Chem 289(17):11704–11714. https://doi.org/10.1074/jbc.M113.502542
Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB (1995) Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med 1(3):237–243. https://doi.org/10.1038/nm0395-237
Mammen AL, Allenbach Y, Stenzel W, Benveniste O (2020) 239th ENMC international workshop: classification of dermatomyositis, Amsterdam, the Netherlands, 14–16 December 2018. Neuromuscul Disord 30(1):70–92. https://doi.org/10.1016/j.nmd.2019.10.005
Martin TC, Šimurina M, Ząbczyńska M, Martinic Kavur M, Rydlewska M, Pezer M, Kozłowska K, Burri A, Vilaj M, Turek-Jabrocka R, Krnjajić-Tadijanović M, Trofimiuk-Müldner M, Ugrina I, Lityńska A, Hubalewska-Dydejczyk A, Trbojevic-Akmacic I, Lim EM, Walsh JP, Pocheć E, Spector TD, Wilson SG, Lauc G (2020) Decreased immunoglobulin G core fucosylation, a player in antibody-dependent cell-mediated cytotoxicity, is associated with autoimmune thyroid diseases. Mol Cell Proteomics 19(5):774–792. https://doi.org/10.1074/mcp.RA119.001860
Matsusaka K, Fujiwara Y, Pan C, Esumi S, Saito Y, Bi J, Nakamura Y, Mukunoki A, Takeo T, Nakagata N, Yoshii D, Fukuda R, Nagasaki T, Tanaka R, Komori H, Maeda H, Watanabe H, Tamada K, Komohara Y, Maruyama T (2021) α(1)-acid glycoprotein enhances the immunosuppressive and protumor functions of tumor-associated macrophages. Cancer Res 81(17):4545–4559. https://doi.org/10.1158/0008-5472.Can-20-3471
Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R (2016) Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res 68(5):689–694. https://doi.org/10.1002/acr.22728
Moremen KW, Tiemeyer M, Nairn AV (2012) Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol 13(7):448–462. https://doi.org/10.1038/nrm3383
Novokmet M, Lukić E, Vučković F, Ðurić Ž, Keser T, Rajšl K, Remondini D, Castellani G, Gašparović H, Gornik O, Lauc G (2014) Changes in IgG and total plasma protein glycomes in acute systemic inflammation. Sci Rep 4:4347. https://doi.org/10.1038/srep04347
Pagan JD, Kitaoka M, Anthony RM (2018) Engineered sialylation of pathogenic antibodies in vivo attenuates autoimmune disease. Cell 172(3):564-577.e513. https://doi.org/10.1016/j.cell.2017.11.041
Pan Y, Zhang L, Zhang R, Han J, Qin W, Gu Y, Sha J, Xu X, Feng Y, Ren Z, Dai J, Huang B, Ren S, Gu J (2021) Screening and diagnosis of colorectal cancer and advanced adenoma by Bionic Glycome method and machine learning. Am J Cancer Res 11(6):3002–3020
Pavić T, Dilber D, Kifer D, Selak N, Keser T, Ljubičić Đ, Vukić Dugac A, Lauc G, Rumora L, Gornik O (2018) N-glycosylation patterns of plasma proteins and immunoglobulin G in chronic obstructive pulmonary disease. J Transl Med 16(1):323. https://doi.org/10.1186/s12967-018-1695-0
Qin W, Zhang Z, Qin R, Han J, Zhao R, Gu Y, Pan Y, Gu J, Ren S (2019) Providing bionic glycome as internal standards by glycan reducing and isotope labeling for reliable and simple quantitation of N-glycome based on MALDI- MS. Anal Chim Acta 1081:112–119. https://doi.org/10.1016/j.aca.2019.07.003
Sato S, Hirakata M, Kuwana M, Suwa A, Inada S, Mimori T, Nishikawa T, Oddis CV, Ikeda Y (2005) Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 52(5):1571–1576. https://doi.org/10.1002/art.21023
Shirakashi M, Nakashima R, Tsuji H, Tanizawa K, Handa T, Hosono Y, Akizuki S, Murakami K, Hashimoto M, Yoshifuji H, Ohmura K, Mimori T (2020) Efficacy of plasma exchange in anti-MDA5-positive dermatomyositis with interstitial lung disease under combined immunosuppressive treatment. Rheumatology 59(11):3284–3292. https://doi.org/10.1093/rheumatology/keaa123
Tokiyama K, Tagawa H, Yokota E, Nagasawa K, Kusaba T, Tsuda Y, Niho Y (1990) Two cases of amyopathic dermatomyositis with fatal rapidly progressive interstitial pneumonitis. Ryumachi 30(3):204–209 (Discussion 209–211)
Tsuchiya N, Endo T, Matsuta K, Yoshinoya S, Aikawa T, Kosuge E, Takeuchi F, Miyamoto T, Kobata A (1989) Effects of galactose depletion from oligosaccharide chains on immunological activities of human IgG. J Rheumatol 16(3):285–290
Varki A, Gagneux P (2012) Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci 1253(1):16–36. https://doi.org/10.1111/j.1749-6632.2012.06517.x
Vreeker GCM, Nicolardi S, Bladergroen MR, van der Plas CJ, Mesker WE, Tollenaar R, van der Burgt YEM, Wuhrer M (2018) Automated plasma glycomics with linkage-specific sialic acid esterification and ultrahigh resolution MS. Anal Chem 90(20):11955–11961. https://doi.org/10.1021/acs.analchem.8b02391
Vučković F, Krištić J, Gudelj I, Teruel M, Keser T, Pezer M, Pučić-Baković M, Štambuk J, Trbojević-Akmačić I, Barrios C, Pavić T, Menni C, Wang Y, Zhou Y, Cui L, Song H, Zeng Q, Guo X, Pons-Estel BA, McKeigue P, Leslie Patrick A, Gornik O, Spector TD, Harjaček M, Alarcon-Riquelme M, Molokhia M, Wang W, Lauc G (2015) Association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome. Arthritis Rheumatol 67(11):2978–2989. https://doi.org/10.1002/art.39273
Zhang Z, Wuhrer M, Holst S (2018) Serum sialylation changes in cancer. Glycoconj J 35(2):139–160. https://doi.org/10.1007/s10719-018-9820-0
Acknowledgements
This work was supported by grants from The National Key R&D Program of China (2022YFC3400803), the National Natural Science Foundation of China (32071276), Greater Bay Area Institute of Precision Medicine (IPM2021C005), and National Postdoctoral Program for Innovative Talents (BX20190076).
Author information
Authors and Affiliations
Contributions
RRZ, LG: carried out the experiments, analysis, interpretation of data and drafted the manuscript. JCS, SWC, JFZ, KWW, JCW: participated in the collection of clinical data. JXG, JL and SFR: conceived the project and revised the intellectual content. All authors contributed to revise the manuscript critically for important intellectual content. All authors read and approved the final manuscript. All authors contributed to drafting the article and revising it critically for important intellectual content. All authors approved the version of the article to be published.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of Interest.
Ethical Approval
This study was approved by Research Ethics Committee of Renji Hospital (ID: IRB #2016-075), Shanghai, China.
Consent to Participate
Written informed consent was obtained from the individual recruited patients of the current study.
Consent for Publication
All the participants approved to publish.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, R., Guo, L., Sha, J. et al. α2,3-Sialylation with Fucosylation Associated with More Severe Anti-MDA5 Positive Dermatomyositis Induced by Rapidly Progressive Interstitial Lung Disease. Phenomics 3, 457–468 (2023). https://doi.org/10.1007/s43657-023-00096-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43657-023-00096-z