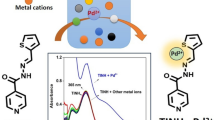
Abstract
A highly selective turn-on sensor for paramagnetic Fe3+ ions based on (E)-N'-((2-aminophenyl)(phenyl)methylene)thiophene-2-carbohydrazide is successfully synthesized. The sensor BPTH is significantly selective and sensitive towards Fe3+ ions over other interfering metal ions especially Cu2+ and Co2+ ions with a lowest limit of recognition 1.48 × 10–7 M. The turn-on sensing mechanism involves enhanced charge transfer. Fe3+ ion forms strong binding with the ligand with a Ka value about 8.23 × 104 M−1 and a 1:1 stoichiometric ratio is confirmed by Job’s plot experiment. With Fe3+ ion, the yellow ligand BPTH change to a green fluorescent and reversible with 1 equivalent of EDTA. Practical application of sensor is demonstrated in real sample analysis.











Similar content being viewed by others
Data availability
Data will be made available on request.
References
Sahoo, S. K., & Crisponi, G. (2019). Recent advances on iron (III) selective fluorescent probes with possible applications in bioimaging. Molecules, 24, 3267.
Zhang, X., Xiong, D., Fu, P., Yun, M., Yang, Q., Jia, M.-M., & Dong, X. (2021). Metal–organic frameworks based on a benzimidazole flexible tetracarboxylic acid: Selective luminescence sensing Fe3+, magnetic behaviors, DFT calculations, and Hirshfeld surface analyses. Applied Organometallic Chemistry, 35, e6431.
Lan, M., Zhao, S., Wei, X., Zhang, K., Zhang, Z., Wu, S., Wang, P., & Zhang, W. (2019). Pyrene-derivatized highly fluorescent carbon dots for the sensitive and selective determination of ferric ions and dopamine. Dyes and Pigments, 170, 107574.
Dinga, Y., & Zhao, C. (2018). A highly selective fluorescent sensor for detection of trivalent metal ions based on a simple Schiff-base. Quimica Nova, 41, 623–627.
Li, B. Y., Li, R., Gao, J., Wang, W.-F., Xie, M.-J., Lu, J., Zheng, F.-K., & Guo, G.-C. (2022). Barium-based coordination polymer: A bi-functional fluorescent sensor for Fe3+ and nitroaromatic molecular detection. Inorganic Chemistry Communications, 137, 109227.
Breuer, W., Ermers, M. J., Pootrakul, P., Abramov, A., Hershko, C., & Cabantchik, Z. I. (2001). Desferrioxamine-chelatable iron, a component of serum non–transferrin-bound iron, used for assessing chelation therapy. Blood, 97, 792–798.
Deng, H., Tian, C., Gao, Z., Chen, S.-W., Li, Y., Zhang, Q., Yu, R., & Wang, J. (2020). Highly luminescent N-doped carbon dots as a fluorescence detecting platform for Fe3+ in solution and living cells. The Analyst, 145, 4931–4936.
Kurz, T., Terman, A., Gustafsson, B., & Brunk, U. T. (2008). Lysosomes in iron metabolism, ageing and apoptosis. Histochemistry and Cell Biology, 129, 389–406.
Botella, H., Stadthagen, G., Lugo-Villarino, G., de Chastellier, C., & Neyrolles, O. (2012). Metallobiology of host-pathogen interactions: An intoxicating new insight. Trends in Microbiology, 20, 106–112.
Xu, H., Zhou, S., Fang, W., & Fan, Y. (2021). Synthesis of N-doped graphene quantum dots from bulk N-doped carbon nanofiber film for fluorescence detection of Fe3+ and ascorbic acid. Fullerenes, Nanotubes, and Carbon Nanostructures, 29, 218–226.
Polatoğlu, B., & Bozkurt, E. (2021). Green synthesis of fluorescent carbon dots from Kumquat (Fortunella margarita) for detection of Fe3+ ions in aqueous solution. Research on Chemical Intermediates, 47, 1865–1881.
Huang, Z., Gao, Y., Huang, Z., Chen, D., Sun, J., & Zhou, L. (2021). Sulfur quantum dots: A novel fluorescent probe for sensitive and selective detection of Fe3+ and phytic acid. Microchemical Journal, 170, 106656.
Kang, S., Han, H., Lee, K., & Kim, K. M. (2022). Ultrasensitive detection of Fe3+ ions using functionalized grapheme. Quantum dots fabricated by a one-step pulsed laser ablation process. ACS Omega, 7, 2074–2081.
Ren, Q., Ga, L., & Ai, J. (2019). Rapid synthesis of highly fluorescent nitrogen-doped graphene quantum dots for effective detection of ferric ions and as fluorescent ink. ACS Omega, 4, 15842–15848.
Kell, D. B. (2009). Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Medical Genomics. https://doi.org/10.1186/1755-8794-2-2
OuYang, H., Gao, Y., & Yuan, Y. (2013). A highly selective rhodamine-based optical-electrochemical multichannel chemosensor for Fe3+. Tetrahedron Letters, 54, 2964–2966.
Fakih, S., Podinovskaia, M., Kong, X., Collins, H. L., Schaible, U. E., & Hider, R. C. (2008). Targeting the lysosome: Fluorescent iron(III) chelators to selectively monitor endosomal/lysosomal labile iron pools. Journal of Medicinal Chemistry, 51, 4539–4552.
Carter, K. P., Young, A. M., & Palmer, A. E. (2014). Fluorescent sensors for measuring metal ions in living systems. Chemical Reviews, 114, 4564–4601.
Sahoo, S. K., Sharma, D., Bera, R. K., Crisponi, G., & Callan, J. F. (2012). Iron(III) selective molecular and supramolecular fluorescent probes. Chemical Society Reviews, 41, 7195–7227.
Brandel, J., Humbert, N., Elhabiri, M., Schalk, I. J., Mislin, G. L., & Albrecht-Gary, A. M. (2012). Pyochelin, a siderophore of Pseudomonas aeruginosa: Physicochemical characterization of the iron(iii), copper(ii) and zinc(ii) complexes. Dalton Transactions, 41, 2820–2834.
Chereddy, N. R., Suman, K., Korrapati, P. S., Thennarasu, S., & Mandal, A. B. (2012). Design and synthesis of rhodamine based chemosensors for the detection of Fe3+ ions. Dyes and Pigments, 95, 606–613.
Wang, X.-Q., Tang, J., Ma, X., Wu, D., & Yang, J. (2021). A water-stable zinc(II)–organic framework as an “on–off–on” fluorescent sensor for detection of Fe3+ and reduced glutathione. CrystEngComm, 23, 1243–1250.
Sahoo, N. K., Jana, G. C., Aktara, M. N., Das, S., Nayim, S. K., Patra, A., Bhattacharjee, P., Bhadra, K., & Hossain, M. (2020). Carbon dots derived from lychee waste: Application for Fe3+ ions sensing in real water and multicolor cell imaging of skin melanoma cells. Mater. Sci. & Eng. C, 108, 110429.
Xu, H., Dong, Y., Wu, Y., Ren, W., Zhao, T., Wang, S., & Gao, J. (2018). An -OH group functionalized MOF for ratiometric Fe3+ Sensing. Journal of Solid State Chemistry, 258, 441–446.
Bar-Ness, E., Hadar, Y., Chen, Y., Shanzer, A., & Libman, J. (1992). NBD-DFO–a probe for Fe uptake from microbial siderophores. Plant Physiology, 99, 1329–1335.
Nesakumar, T., Edison, J. I., Atchudan, R., Shim, J.-J., Kalimuthu, S., Ahn, B.-C., & Lee, Y. R. (2016). Turn-off fluorescence sensor for the detection of ferric ion in water using green synthesized N-doped carbon dots and its bio-imaging. Journal of Photochemistry and Photobiology B: Biology., 158, 235–242.
Abdullah, M., Abidin, Z. Z., Sobri, S., Rashid, S. A., Mahdi, M. A., & Ibrahim, N. A. (2020). Fluorescent recognition of Fe3+ in acidic environment by enhanced-quantum yield N-doped carbon dots: Optimization of variables using central composite design. Science and Reports, 10, 11710.
Yang, Z., She, M., Yin, B., Cui, J., Zhang, Y., Sun, W., Li, J., & Shi, Z. (2012). Three rhodamine-based “off-on” chemosensors with high selectivity and sensitivity for Fe3+ imaging in living cells. Journal of Organic Chemistry, 77, 1143–1147.
Puthiyedath, T., & Bahulayan, D. (2018). A Click derived triazole-coumarin derivative as fluorescence on-off PET based sensor for Ca2+ and Fe3+ ions. Sensors and Actuators, B: Chemical Sensors and Materials, 272, 110–117.
Sarih, N. M., Ciupa, A., Moss, S., Myers, P., Slater, A. G., Abdullah, Z., Tajuddin, H. A., & Maher, S. (2020). Furo[3,2-c]coumarin-derived Fe3+ selective fluorescence sensor: Synthesis, fluorescence study and application to water analysis. Science and Reports, 10, 7421.
Nadgir, A., & Sidarai, A. H. (2021). Photophysical investigation of a benzimidazole derivative and its applications in selective detection of Fe3+, thermosensing and logic gates. Journal of Fluorescence, 31, 1503–1512.
Vanjare, B. D., Mahajan, P. G., Hong, S.-K., & Lee, K. H. (2018). Discriminating chemosensor for detection of Fe3+ in aqueous media by fluorescence quenching methodology. Bulletin of the Korean Chemical Society, 39, 631–637.
Patil, N. B., Patil, U. D., Patil, P. A., Bothra, S., Sahoo, S. K., Sehlangia, S., Pradeep, C. P., Patil, A. A., & Patil, S. R. (2019). A Fused benzothiazolo-pyrimidine-based chemosensor for selective optical detection of Fe3+ and I− ions in aqueous media. ChemistrySelect, 4, 4185–4189.
Zhang, Y. M., Chen, X. P., Liang, G. Y., Zhong, K. P., Yao, H., Wei, T. B., & Lin, Q. (2018). A water-soluble fluorescent chemosensor based on Asp functionalized naphthalimide for successive detection Fe3+ and H2PO4-. Canadian Journal of Chemistry., 96, 1–8.
Sayed, A., Othman, I. M. M., Hamam, M., Gomaa, H., Gadallah, M. I., Mostfa, M. A., Ali, H. R. H., Emran, M. Y., Abdel-Hakim, M., & Mahross, M. H. (2021). A novel fluorescent sensor for fast and highly selective turn-off detection of Fe3+ in water and pharmaceutical samples using synthesize d azopyrazole-benzenesulfonamide derivative. Journal of Molecular Structure, 1225, 129175.
Cai, X., Ye, J., Zhou, Q., Yan, Z., & Li, K. (2020). A novel AIE “on-off-on” fluorescence sensor for highly selective and sensitive sequential detection of Fe3+ and HSO3− in foods. Microchemical Journal., 159, 105419.
Zhang, B., Liu, H., Wu, F., Hao, G., Chen, Y., Tan, C., Tan, Y., & Jiang, Y. (2017). A dual-response quinoline-based fluorescent sensor for the detection of Copper (II) and Iron(III) ions in aqueous medium. Sensors and Actuators, B: Chemical Sensors and Materials, 243, 765–774.
Zhu, X., Duan, Y., Li, P., Fan, H., Han, T., & Huang, X. (2019). A highly selective and instantaneously responsive Schiff base fluorescent sensor for the “turn-off” detection of iron(III), iron(II), and copper(II) ions. Analytical Methods, 11, 642–647.
Darole, R. S., DenzilBritto, C., Mukherjee, A., Gonnade, R. G., Sekar, K., & Senthilkumar, B. (2020). Anthrone-spirolactam and quinoline hybrid based sensor for selective fluorescent detection of Fe3+ ions. Applied Organometallic Chemistry, 34, e5867.
Tamil Selvan, G., Varadaraju, C., Tamil Selvan, R., Enoch, I. V. M. V., & Selvakumar, P. M. (2018). On/Off fluorescent chemosensor for selective detection of divalent iron and copper ions: Molecular logic operation and protein binding. ACS Omega, 3, 7985–7992.
Li, B., Gu, X., Wang, M., Liu, X., & Xu, K. (2021). A novel “off-on-off” fluorescent probe for sensing of Fe3+ and F- successively in aqueous solution and its application in cells. Dyes and Pigments, 194, 109637.
Wang, Y., Pak, Y. L., & Xu, Q. (2021). A selective fluorescent probe for ferric ion based on rhodamine 6G. Bulletin of the Korean Chemical Society, 42, 262–264.
Wang, K. P., Zheng, W. J., Lei, Y., Zhang, S. J., Zhang, Q., Chen, S., & Hu, Z. Q. (2019). A thiophene-rhodamine dyad as fluorescence probe for ferric ion and its application in living cells imaging. Journal of Fluorescence, 208, 468–474.
Wang, P., Liu, X., Fu, J., Chang, Y., Yang, L., & Xu, K. (2018). Synthesis and fluorescence spectral studies of novel quinolylbenzothiazole-based sensors for selective detection of Fe3+ ion. Canadian Journal of Chemistry, 96, 1–7.
Song, F., Yang, C., Liu, H., Gao, Z., Zhu, J., Bao, X., & Kan, C. (2019). Dual-binding pyridine and rhodamine B conjugate derivatives as fluorescent chemosensors for Ferric ion in aqueous media and living cells. The Analyst, 144, 3094–3102.
Kouser, R., Zehra, S., Khan, R. A., Alsalme, A., Arjmand, F., & Tabassum, S. (2021). “Turn-on” benzophenone based fluorescence and colorimetric sensor for the selective detection of Fe2+ in aqueous media: Validation of sensing mechanism by spectroscopic and computational studies. Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy, 247, 119156.
Jadhav, A. G., Shinde, S. S., Lanke, S. K., & Sekar, N. (2017). Benzophenone based fluorophore for selective detection of Sn2+ ion: Experimental and theoretical study. Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy, 174, 291–296.
Sinha, S., Koner, R. R., Kumar, S., Mathew, J., Monisha, P. V., Kazia, I., & Ghosh, S. (2013). Imine containing benzophenone scaffold as an efficient chemical device to detect selectively Al3+. RSC Advances, 3, 345–351.
Nandhini, T., Kaleeswaran, P., & Pitchumani, K. (2016). A highly selective, sensitive and “turn-on” fluorescent sensor for the paramagnetic Fe3+ ion. Sensors and Actuators, B: Chemical Sensors and Materials, 230, 199–205.
Jo, T. G., Bok, K. H., Han, J., Lim, M. H., & Kim, C. (2017). Colorimetric detection of Fe3+ and Fe2+ and sequential fluorescent detection of Al3+ and pyrophosphate by an imidazole-based chemosensor in a near-perfect aqueous solution. Dyes and Pigments, 139, 136–147.
He, Y., Yin, J., & Wang, G. (2018). New selective “on-off” fluorescence chemosensor based on carbazole Schiff base for Fe3+ detection. Chem. of Heterocycl. Compd., 42, 146–152.
Gong, X., Ding, X., Jiang, N., Zhong, T., & Wang, G. (2020). Benzothiazole-based fluorescence chemosensors for rapid recognition and “turn-off” fluorescence detection of Fe3+ ions in aqueous solution and in living cells. Microchemical Journal, 152, 104351.
Hu, S., Zhang, S., Gao, C., Xu, C., & Gao, Q. (2013). A new selective fluorescent sensor for Fe3+ based on a pyrazoline derivative. Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy, 113, 325–331.
Sasan, S., Chopra, T., Gupta, A., Tsering, D., Kapoor, K. K., & Parkesh, R. (2022). Fluorescence “Turn-Off” and colorimetric sensor for Fe2+, Fe3+, and Cu2+ ions based on a 2,5,7-triarylimidazopyridine scaffold. ACS Omega, 7, 11114–11125.
Rasin, P., Mathew, M. M., Manakkadan, V., Palakkeezhillam, V. N. V., & Sreekanth, A. (2022). A highly fluorescent pyrene-based sensor for selective detection of Fe3+ ion in aqueous medium: computational investigations. Journal of Fluorescence., 32, 1229–1238.
You, G. R., Park, G. J., Lee, S. A., Ryu, K. Y., & Kim, C. (2015). Chelate-type Schiff base acting as a colorimetric sensor for iron in aqueous solution. Sensors and Actuators, B: Chemical Sensors and Materials, 215, 188–195.
Acknowledgements
The author Dr. C. Denzil Britto is very thankful for University Post-Doctoral Fellowship (Order No. PU/CDC/UPDF/AD-3/003248/2021-1 & PU/AD3/UPDF Continuation Order/22F47567/2022) by Periyar University, Salem, 636011, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript declare that there is no conflict of interest in publishing our article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Christopher Leslee, D.B., Madheswaran, B., Gunasekaran, J. et al. Iminobenzophenone-thiophen hydrazide schiff base: a selective turn on sensor for paramagnetic Fe3+ ion and application in real sample analysis. Photochem Photobiol Sci 22, 1933–1943 (2023). https://doi.org/10.1007/s43630-023-00422-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-023-00422-4