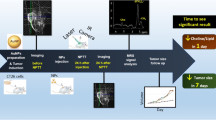
Abstract
Background
Photo-thermal therapy (PTT) has been at the center of attention as a new method for cancer treatment in recent years. It is important to predict the response to treatment in the PTT procedure. Using magnetic resonance spectroscopy (MRS) can be considered a novel technique in evaluating changes in metabolites resulted from PTT.
Methods
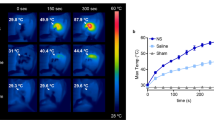
In the present project, we conducted an in vivo study to assess the efficacy of 1H-MRS as a noninvasive technique to evaluate the response to treatment in the early hours following PTT. The BALB/c mice subcutaneously bearing tumor cells (CT26 cell line) were scanned by 1H-MRS before and after PTT. Iron oxide-gold core–shell (Fe3O4@Au) as PTT agent was injected into intra-peritoneal at first and then irradiated by NIR laser. Single-voxel Point RESolved Spectroscopy (PRESS) sequence (TE = 144) was used, and metabolites alternations were evaluated by the non-parametric Wilcoxon test. Besides, Nanoparticle (NP) relaxometry was conducted for negative contrast agents’ potentials.
Results
MRS choline (Cho) peak dramatically reduced 24 h post-PTT (p = 0.01) and lipid peak as a marker for necrosis of tumor elevated (p = 0.01) just in group 3 (NPs injection + laser irradiation) 24 h after the procedure.
Conclusion
1H-MRS showed its potential as a method in detecting the changes in metabolites and revealing the outcome accurately. Response to photo-thermal therapy evaluation was achievable only one day after PTT and proved by a 10-day follow-up of the tumor size. Iron oxide-gold core–shell can also be used as a negative contrast agent in MRI images during therapy.









Similar content being viewed by others
References
Pearce, A., Haas, M., Viney, R., et al. (2017). Incidence and severity of self-reported chemotherapy side effects in routine care: a prospective cohort study. PLoS ONE, 12(10), 0184360. https://doi.org/10.1371/journal.pone.0184360.
Taylor, C. W., & Kirby, A. M. (2015). Cardiac side-effects from breast cancer radiotherapy. Clinical Oncology, 27(11), 621–629. https://doi.org/10.1016/j.clon.2015.06.007.
Bruheim, K., Guren, M. G., Skovlund, E., Hjermstad, M. J., Dahl, O., Frykholm, G., et al. (2010). Late side effects and quality of life after radiotherapy for rectal cancer. International Journal of Radiation Oncology Biology Physics., 76(4), 1005–1011. https://doi.org/10.1016/j.ijrobp.2009.03.010.
Zhou, F., Wu, S., Wu, B., Chen, W. R., & Xing, D. (2011). Mitochondria-targeting single-walled carbon nanotubes for cancer photothermal therapy. Small (Weinheim an der Bergstrasse, Germany), 7(19), 2727–2735. https://doi.org/10.1002/smll.201100669.
Vines, J. B., Yoon, J. H., Ryu, N. E., Lim, D. J., & Park, H. (2019). Gold nanoparticles for photothermal cancer therapy. Frontiers in Chemistry. https://doi.org/10.3389/fchem.2019.00167.
Liu, Y., Bhattarai, P., Dai, Z., & Chen, X. (2019). Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chemical Society Review, 48(7), 2053–2108. https://doi.org/10.1039/c8cs00618k.
Montazerabadi, A., Beik, J., Irajirad, R., et al. (2019). Folate-modified and curcumin-loaded dendritic magnetite nanocarriers for the targeted thermo-chemotherapy of cancer cells. Artifical Cells, Nanomedicine, and Biotechnology, 47(1), 330–340. https://doi.org/10.1080/21691401.2018.1557670.
Huang, X., Jain, P. K., El-Sayed, I. H., & El-Sayed, M. A. (2007). Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine (London)., 2(5), 681–693. https://doi.org/10.2217/17435889.2.5.681.
Cai, W., Gao, T., Hong, H., & Sun, J. (2008). Applications of gold nanoparticles in cancer nanotechnology. Nanotechnology Science and Applications., 12(1), 17–32. https://doi.org/10.2147/nsa.s3788.
Ma, X., Tao, H., Yang, K., et al. (2012). A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. Nano Research, 5, 199–212. https://doi.org/10.1007/s12274-012-0200-y.
Zhang, M., Cao, Y., Wang, L., Ma, Y., Tu, X., & Zhang, Z. (2015). Manganese doped iron oxide theranostic nanoparticles for combined T1 magnetic resonance imaging and photothermal therapy. ACS Applied Materials Interfaces., 7(8), 4650–4658. https://doi.org/10.1021/am5080453.
Abed, Z., Beik, J., Laurent, S., et al. (2019). Iron oxide-gold core-shell nano-theranostic for magnetically targeted photothermal therapy under magnetic resonance imaging guidance. Journal of Cancer Research and Clinical Oncology., 145(5), 1213–1219. https://doi.org/10.1007/s00432-019-02870-x.
Montazerabadi, A. R., Oghabian, M. A., Irajirad, R., Muhammadnejad, S., Ahmadvand, D., Delavari, H. H., & Mahdavi, S. R. (2015). Development of gold-coated magnetic nanoparticles as a potential MRI contrast agent. NANO, 10(04), 1550048.
Jing, L., Liang, X., Lin, L., et al. (2014). Mn-porphyrin conjugated Au nanoshells encapsulating doxorubicin for potential magnetic resonance imaging and light triggered synergistic therapy of cancer. Theranostics., 4(9), 858–871. https://doi.org/10.7150/thno.8818.
Wang, H., Mu, Q., Revia, R., et al. (2018). Iron oxide-carbon core-shell nanoparticles for dual-modal imaging-guided photothermal therapy. Journal of Controlled Release: Official Journal of the Controlled Release Society., 289, 70–78. https://doi.org/10.1016/j.jconrel.2018.09.022.
Estelrich, J., & Busquets, M. A. (2018). Iron oxide nanoparticles in photothermal therapy. Molecules, 23(7), 1567. https://doi.org/10.3390/molecules23071567.
Mirrahimi, M., Abed, Z., Beik, J., Shiri, I., Dezfuli, A. S., Mahabadi, V. P., et al. (2019). A thermo-responsive alginate nanogel platform co-loaded with gold nanoparticles and cisplatin for combined cancer chemo-photothermal therapy. Pharmacological Research., 1(143), 178–185. https://doi.org/10.1016/j.phrs.2019.01.005.
Zhou, J., Lu, Z., Zhu, X., et al. (2013). NIR photothermal therapy using polyaniline nanoparticles. Biomaterials, 34(37), 9584–9592. https://doi.org/10.1016/j.biomaterials.2013.08.075.
Rostami, A., & Sazgarnia, A. (2019). Gold nanoparticles as cancer theranostic agents. Nanomedicine Journal., 6(3), 147–160. https://doi.org/10.22038/nmj.2019.06.00001.
Kim, M. J., Lee, S. J., Lee, J. H., et al. (2012). Detection of rectal cancer and response to concurrent chemoradiotherapy by proton magnetic resonance spectroscopy. Magnetic Resonance Imaging., 30(6), 848–853. https://doi.org/10.1016/j.mri.2012.02.013.
Zhou, J., Qiao, P. G., Zhang, H. T., Li, G. J., & Jiang, Z. F. (2018). Predicting neoadjuvant chemotherapy in nonconcentric shrinkage pattern of breast cancer using 1H-magnetic resonance spectroscopic imaging. Journal of Computer Assisted Tomography., 42(1), 12–18. https://doi.org/10.1097/rct.0000000000000647.
Chae, W. H., Niesel, K., Schulz, M., Klemm, F., Joyce, J. A., Prümmer, M., et al. (2019). evaluating magnetic resonance spectroscopy as a tool for monitoring therapeutic response of whole brain radiotherapy in a mouse model for breast-to-brain metastasis. Frontiers in Oncology., 9, 1324. https://doi.org/10.3389/fonc.2019.01324.
Toussaint, M., Pinel, S., Auger, F., Durieux, N., Thomassin, M., Thomas, E., et al. (2017). Proton MR spectroscopy and diffusion MR imaging monitoring to predict tumor response to interstitial photodynamic therapy for glioblastoma. Theranostics., 7(2), 436–451. https://doi.org/10.7150/thno.17218.
Kwock, L., Smith, J. K., Castillo, M., et al. (2006). Clinical role of proton magnetic resonance spectroscopy in oncology: brain, breast, and prostate cancer. The Lancet Oncology., 7(10), 859–868. https://doi.org/10.1016/S1470-2045(06)70905-6.
Oh, J., Henry, R. G., Pirzkall, A., et al. (2004). Survival analysis in patients with glioblastoma multiforme: predictive value of choline-to-N-acetylaspartate index, apparent diffusion coefficient, and relative cerebral blood volume. Journal of Magnetic Resonance Imaging., 19(5), 546–554. https://doi.org/10.1002/jmri.20039.
Yu, T. G., Feng, Y., Feng, X. Y., Dai, J. Z., Qian, H. J., & Huang, Z. (2015). Prognostic factor from MR spectroscopy in rat with astrocytic tumour during radiation therapy. The British Journal of Radiology., 88(1045), 20140418. https://doi.org/10.1259/bjr.20140418.
Chen, H., Zhang, X., Dai, S., Ma, Y., Cui, S., Achilefu, S., & Gu, Y. (2013). Multifunctional gold nanostar conjugates for tumor imaging and combined photothermal and chemo-therapy. Theranostics., 3(9), 633–649. https://doi.org/10.7150/thno.6630.
Kim, J., Park, S., Lee, J. E., et al. (2006). Designed fabrication of multifunctional magnetic gold nanoshells and their application to magnetic resonance imaging and photothermal therapy. Angewandte Chemie International Edition England., 45(46), 7754–7758. https://doi.org/10.1002/anie.200602471.
Eyvazzadeh, N., Shakeri-Zadeh, A., Fekrazad, R., Amini, E., Ghaznavi, H., & Kamran, K. S. (2017). Gold-coated magnetic nanoparticle as a nanotheranostic agent for magnetic resonance imaging and photothermal therapy of cancer. Lasers in Medical Science., 32(7), 1469–1477. https://doi.org/10.1007/s10103-017-2267-x.
Dong, W., Li, Y., Niu, D., et al. (2011). Facile synthesis of monodisperse superparamagnetic Fe3O4 Core@hybrid@Au shell nanocomposite for bimodal imaging and photothermal therapy. Advanced Materials., 23(45), 5392–5397. https://doi.org/10.1002/adma.201103521.
Ji, X., Shao, R., Elliott, A. M., Stafford, R. J., Esparza-Coss, E., Bankson, J. A., et al. (2007). Bifunctional gold nanoshells with a superparamagnetic iron oxide-silica core suitable for both MR imaging and photothermal therapy. The Journal of Physical Chemistry C, Nanomaterials and Interfaces, 111(17), 6245–6251. https://doi.org/10.1021/jp0702245.
Verma, A., Kumar, I., Verma, N., Aggarwal, P., & Ojha, R. (2016). Magnetic resonance spectroscopy - Revisiting the biochemical and molecular milieu of brain tumors. BBA Clinical., 5, 170–178. https://doi.org/10.1016/j.bbacli.2016.04.002.
Mader, I., Rauer, S., Gall, P., & Klose, U. (2008). H MR Spectroscopy of inflammation, infection and ischemia of the brain. European Journal of Radiology., 67(2), 250–257. https://doi.org/10.1016/j.ejrad.2008.02.033.
García-Figueiras, R., Baleato-González, S., Padhani, A. R., et al. (2018). Advanced imaging techniques in evaluation of colorectal cancer. Radiographics., 38(3), 740–765. https://doi.org/10.1148/rg.2018170044.
Zhou, Z., Sun, Y., Shen, J., Wei, J., Yu, C., Kong, B., et al. (2014). Iron/iron oxide core/shell nanoparticles for magnetic targeting MRI and near-infrared photothermal therapy. Biomaterials, 35, 7470–7478. https://doi.org/10.1016/j.biomaterials.2014.04.063.
Jordan, K. W., Nordenstam, J., Lauwers, G. Y., et al. (2009). Metabolomic characterization of human rectal adenocarcinoma with intact tissue magnetic resonance spectroscopy. Diseases of the Colon and Rectum., 52(3), 520–525. https://doi.org/10.1007/DCR.0b013e31819c9a2c.
García-Figueiras, R., Baleato-González, S., Padhani, A. R., Marhuenda, A., Luna, A., Alcalá, L., et al. (2016). Advanced imaging of colorectal cancer: from anatomy to molecular imaging. Insights into Imaging., 7(3), 285–309. https://doi.org/10.1007/s13244-016-0465-x.
Ghaznavi, H., Hosseini-Nami, S., Kamrava, S. K., Irajirad, R., Maleki, S., Shakeri-Zadeh, A., & Montazerabadi, A. (2018). Folic acid conjugated PEG coated gold–iron oxide core–shell nanocomplex as a potential agent for targeted photothermal therapy of cancer. Artificial Cells, Nanomedicine, and Biotechnology., 46(8), 1594–1604. https://doi.org/10.1080/21691401.2017.1384384.
García-Figueiras, R., Baleato-González, S., Padhani, A. R., et al. (2016). Advanced imaging of colorectal cancer: from anatomy to molecular imaging. Insights Imaging., 2016(7), 285–309. https://doi.org/10.1007/s13244-016-0465-x.
Dzik-Jurasz, A. S., Murphy, P. S., George, M., et al. (2002). Human rectal adenocarcinoma: demonstration of 1H-MR spectra in vivo at 1.5 T. Magnetic Resonance in Medicine., 47(4), 809–811. https://doi.org/10.1002/mrm.10108.
Mun, C. W., Cho, J. Y., Shin, W. J., et al. (2004). Ex vivo proton MR spectroscopy (1H-MRS) for evaluation of human gastric carcinoma. Magnetic Resonance Imaging., 22(6), 861–870. https://doi.org/10.1016/j.mri.2004.01.045.
García-Figueiras, R., Baleato-González, S., Padhani, A. R., et al. (2016). Proton magnetic resonance spectroscopy in oncology: the fingerprints of cancer? Diagnostic and Interventional Radiology., 22(1), 75–89. https://doi.org/10.5152/dir.2015.15009.
Aboagye, E. O., & Bhujwalla, Z. M. (1999). Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Research, 59(1), 80–84.
Mountford, C. E., Saunders, J. K., May, G. L., et al. (1986). Classification of human tumours by high-resolution magnetic resonance spectroscopy. Lancet, 1(8482), 651–653. https://doi.org/10.1016/s0140-6736(86)91727-7.
Lee, J. H., Cho, K. S., Kim, Y. M., et al. (1998). Localized in vivo 1H nuclear MR spectroscopy for evaluation of human uterine cervical carcinoma. AJR. American Journal of Roentgenology, 170(5), 1279–1282. https://doi.org/10.2214/ajr.170.5.9574601.
Castillo, M., Kwock, L., & Mukherji, S. K. (1996). Clinical applications of proton MR spectroscopy. AJNR. American Journal of Neuroradiology, 17(1), 1–15.
Kimura, T., Sako, K., Gotoh, T., Tanaka, K., & Tanaka, T. (2001). In vivo single-voxel proton MR spectroscopy in brain lesions with ring-like enhancement. NMR in Biomedicine., 14(6), 339–349. https://doi.org/10.1002/nbm.711.
Beik, J., Asadi, M., Khoei, S., et al. (2019). Simulation-guided photothermal therapy using MRI-traceable iron oxide-gold nanoparticle. Journal of Photochemistry and Photobiology B: Biology., 199, 111599. https://doi.org/10.1016/j.jphotobiol.2019.111599.
Asadi, M., Beik, J., Hashemian, R., et al. (2019). MRI-based numerical modeling strategy for simulation and treatment planning of nanoparticle-assisted photothermal therapy. Physica Medica., 66, 124–132. https://doi.org/10.1016/j.ejmp.2019.10.002.
Acknowledgements
The present study was derived from an MSc thesis (Research project code: 981175) which has been supported by the Deputy of Research and Technology of Mashhad University of Medical Sciences, Mashhad, Iran. Authors would like to acknowledge Iranian National Brain Mapping Laboratory (NBML), Tehran, Iran, for providing data acquisition service for this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Ehsani, S., Saatchian, E., Sarikhani, A. et al. 1H-MRS application in the evaluation of response to photo-thermal therapy using iron oxide-gold core-shell nanoparticles, an in vivo study. Photochem Photobiol Sci 20, 245–254 (2021). https://doi.org/10.1007/s43630-021-00012-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-021-00012-2