Abstract
Purpose
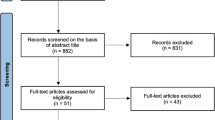
Antimicrobial cement spacer (ACS) placement has been a cornerstone of two-stage management of prosthetic hip and knee infection. Pharmacokinetic modelling has described peak systemic antibiotic concentrations within the first 24–48 h post-operatively, followed by rapid clearance. A few studies have, however, identified detectable tobramycin levels in patients with a post-operative decline in creatinine clearance. Our study sought to determine how frequently detectable serum tobramycin levels occurred within the first 72 h following ACS placement in all patients regardless of baseline or subsequent changes in renal function, whether these levels correlated with tobramycin spacer dosage, creatinine clearance, or potential nephrotoxicity risk factors, and whether any patients developed acute kidney injury within the 14-day post-operative period.
Methods
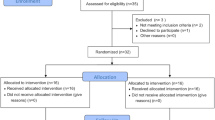
We prospectively enrolled patients with prosthetic hip or knee infections and subsequent ACS placement from October 2017 to February 2020. Patient comorbidities (chronic kidney disease, diabetes mellitus, chronic liver disease, chronic obstructive pulmonary disease, and atrial fibrillation), Charleston Comorbidity Index score, risk factors for post-operative nephrotoxicity (perioperative hypotension and nephrotoxic agent receipt), total tobramycin dosage, post-operative days 1 and 3 serum tobramycin concentrations, and serum creatinine and creatinine clearance throughout a 14-day post-operative period were recorded.
Results
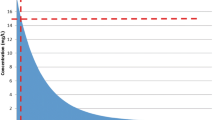
A total of 20 patients were enrolled, comprising 20 spacers with a median total tobramycin dosage of 4.80 g with an interquartile range (IQR) of 4.13–7.20 g. Thirteen patients had a median detectable post-operative day 1 serum tobramycin concentration of 0.80 (IQR 0.50–1.60) mcg/mL. Five of these 13 patients had a median detectable post-operative day 3 serum tobramycin concentration of 0.80 (IQR 0.50–1.10) mcg/mL. A correlation was not found between serum tobramycin drug levels and patient comorbidities, receipt of nephrotoxic medications, or baseline and subsequent post-operative creatinine clearance up to day 14.
Conclusion
The majority of patients who underwent tobramycin ACS placement had detectable serum tobramycin levels in the immediate post-operative period, but most reached undetectable levels within 72 h. There were no reliable perioperative predictors of detectable drug levels.

Similar content being viewed by others
Code Availability
Not applicable.
Data Availability
Not applicable.
References
Phillips, J., Crane, T., Noy, M., Elliott, T., & Grimer, R. (2006). The incidence of deep prosthetic infections in a specialist orthopaedic hospital. The Journal of Bone and Joint Surgery British, 88-B, 943–948.
Edelstein, A., Okroj, K., Rogers, T., Della Valle, C., & Sporer, M. (2017). Systemic absorption of antibiotics from antibiotic-loaded cement spacers for the treatment. Journal of Arthroplasty, 33(3), 835–839.
Kendoff, D. O., Gehrke, T., Stangenberg, P., Frommelt, L., & Bosebeck, H. (2016). Bioavailability of gentamicin and vancomycin released from an antibiotic containing bone cement in patients undergoing a septic one-stage total hip arthroplasty (THA) revision: A monocentric open clinical trial. Hip International, 26(1), 90–96.
Bejon, P., Berendt, A., Atkins, B., Green, N., Parry, H., Masters, S., Mclardy-Smith, P., Gundle, R., & Byren, I. (2010). Two-stage revision for prosthetic joint infection: Predictors of outcome and the role for preimplantation microbiology. Journal of Antimicrobial Chemotherapy, 65, 569–575.
Bertazzoni, M. E., Benini, A., Samaila, E., Bondi, M., & Magnan, B. (2015). Antimicrobial activity of gentamicin and vancomycin combination in joint fluids after antibiotic-loaded cement spacer implantation in two-stage revision surgery. Journal of Chemotherapy, 27(1), 17–24.
Hsieh, P. H., Huang, K. C., & Tai, C. L. (2009). Liquid gentamicin in bone cement spacers: In vivo antibiotic release and systemic safety in two-stage revision of infected hip arthroplasty. Journal of Trauma, 66(3), 804–808.
Sterling, G. J., Crawford, S., Potter, J. H., Koerbin, G., & Crawford, R. (2003). The pharmacokinetics of Simplex-tobramycin bone cement. The Journal of Bone and Joint Surgery, 85-B, 646–649.
Luu, A., Syed, F., Raman, G., et al. (2013). Two-stage arthroplasty for prosthetic joint infection: A systematic review of acute kidney injury, systemic toxicities and infection control. Journal of Arthroplasty, 28(9), 1490–1498.
Aeng, E. S., Shalansky, K. F., Lau, T. T., et al. (2015). Acute kidney injury with tobramycin-impregnated bone cement spacers in prosthetic joint infections. Annals of Pharmacotherapy, 49(11), 1207–1213.
Noto, M. J., et al. (2014). Detectable serum tobramycin levels in patients with renal dysfunction and recent placement of antibiotic-impregnated cement knee or hip spacers. Clinical Infectious Diseases, 58, 1783–1784.
Bellomo, R., Ronco, C., Kellum, J. A., Mehta, R. L., & Palevsky, P. (2004). Acute dialysis quality initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (ADQI) group. Critical Care, 8(4), R204–R212. https://doi.org/10.1186/cc2872
Menge, T. J., et al. (2012). Acute kidney injury after placement of an antibiotic-impregnated cement spacer during revision total knee arthroplasty. The Journal of Arthroplasty, 27, 1221–1229.
Wu, I., Marin, E., Kashgarian, M., Ian, M., & Brewster, U. C. (2009). A case of an acute kidney injury secondary to an implanted aminoglycoside. Kidney International, 75(10), 1109–1112.
Funding
The Allegheny Science and Research Institute at Allegheny Health Network provided a research grant (19010109 AHN Research Initiatives) which reimbursed the sole expense of this study—the cost of drawing and processing serum tobramycin levels.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Ethical Approval
This study was approved by the Allegheny Health Network IRB under a “full review” process, prior to subject enrollment, and was renewed annually for the duration of the study.
Informed Consent
For this type of study, informed consent is not required.
Consent to Participate
All subjects provided informed consent prior to study enrollment.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Como, J.D., Abdulmassih, R., Guarascio, A.J. et al. Systemic Absorption Resulting from Tobramycin-Loaded Antibiotic Cement Spacers Used in the Treatment of Prosthetic Joint Infection. JOIO 58, 144–150 (2024). https://doi.org/10.1007/s43465-023-01075-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43465-023-01075-2