Abstract
Introduction
We investigated the safety, efficacy, functional, and clinical outcomes of intra-osseous implantation of mechanically isolated, autologous stromal vascular fraction (SVF), an Australian patented direct ultrasonication technology (Sahaj Therapy®) in osteonecrosis of the femoral head (ONFH).
Materials and Methods
A total of 32 cases of ONFH were enrolled in the study after confirming with an MRI of the affected hip. All cases were treated with an intra-osseous autologous SVF implantation [4–5 cc with the cellular dosage of 8.0 × 107 cells with a viability of > 85% SVF cells] on the same surgical sitting. All the cases were followed up clinically, functionally, and radiologically at regular intervals. A comparison of mean HOOS scores at different follow-ups was done using Paired ‘t’-test. A P value of < 0.05 was considered significant.
Results
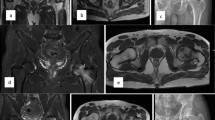
In our study, male preponderance was seen (53.1%). According to the modified Ficat and Arlet classification, the most common grade of ONFH was grade 2 [right: 25 hips and left: 25 hips]. There was a statistically significant improvement in the mean HOOS score of the right hip (n = 10) and left hip (n = 9) from preoperative time till 72 months (P < 0.05). The follow-up MRI of the affected hips shows improved osteogenesis without any further worsening of the contour of the femoral head. No adverse effects were seen in any of the study participants.
Conclusion
For individuals with ONFH, treated with intra-osseous autologous SVF implantation in the same surgical procedure is an innovative and promising treatment modality. Even after 6 years of follow-up, the study participants with ONFH have shown good clinical and functional outcomes with autologous SVF.







Similar content being viewed by others
References
Lespasio, M. J., Sodhi, N., & Mont, M. A. (2019). Osteonecrosis of the hip: A primer. The Permanente Journal, 23, 18–100. https://doi.org/10.7812/TPP/18-100
Moghamis, I., Alhammoud, A. A., Kokash, O., & Alhaneedi, G. A. (2021). The outcome of hyperbaric oxygen therapy versus core decompression in the non-traumatic avascular necrosis of the femoral head: Retrospective cohort study. Annals of Medicine and Surgery, 62, 450–454. https://doi.org/10.1016/j.amsu.2021.01.084
Chang, C. C., Greenspan, A., & Gershwin, M. E. (1993). Osteonecrosis: Current perspectives on pathogenesis and treatment. Seminars in Arthritis and Rheumatism, 23(1), 47–69. https://doi.org/10.1016/s0049-0172(05)80026-5
Marker, D. R., Seyler, T. M., Ulrich, S. D., Srivastava, S., & Mont, M. A. (2008). Do modern techniques improve core decompression outcomes for hip osteonecrosis? Clinical Orthopaedics and Related Research, 466(5), 1093–1103. https://doi.org/10.1007/s11999-008-0184-9
Jones, K. B., Seshadri, T., Krantz, R., Keating, A., & Ferguson, P. C. (2008). Cell-based therapies for osteonecrosis of the femoral head. Biology of Blood and Marrow Transplantation, 14(10), 1081–1087. https://doi.org/10.1016/j.bbmt.2008.06.017
Cardozo, J. B., Andrade, D. M. S., & Santiago, M. B. (2008). The use of bisphosphonate in the treatment of avascular necrosis: A systematic review. Clinical Rheumatology, 27(6), 685–688. https://doi.org/10.1007/s10067-008-0861-9
Lieberman, J. R., Berry, D. J., Mont, M. A., Aaron, R. K., Callaghan, J. J., Rajadhyaksha, A. D., et al. (2003). Osteonecrosis of the hip: Management in the 21st century. Instructional Course Lectures, 52, 337–355.
Mont, M. A., & Hungerford, D. S. (1995). Non-traumatic avascular necrosis of the femoral head. The Journal of Bone and Joint Surgery. American Volume, 77(3), 459–474. https://doi.org/10.2106/00004623-199503000-00018
Mont, M. A., Jones, L. C., & Hungerford, D. S. (2006). Nontraumatic osteonecrosis of the femoral head: Ten years later. The Journal of Bone and Joint Surgery. American Volume, 88(5), 1117–1132. https://doi.org/10.2106/JBJS.E.01041
Fan, M., Peng, J., Qin, L., & Lu, S. (2011). Experimental animal models of osteonecrosis. Rheumatology International, 31(8), 983–994. https://doi.org/10.1007/s00296-011-1819-9
Hernigou, P., Poignard, A., Nogier, A., & Manicom, O. (2004). Fate of very small asymptomatic stage-I osteonecrotic lesions of the hip. The Journal of Bone and Joint Surgery. American Volume, 86(12), 2589–2593. https://doi.org/10.2106/00004623-200412000-00001
Mankin, H. J. (1992). Nontraumatic necrosis of bone (osteonecrosis). The New England Journal of Medicine, 326(22), 1473–1479. https://doi.org/10.1056/NEJM199205283262206
Jones, J. P. (1999). Coagulopathies and osteonecrosis. Acta Orthopaedica Belgica, 65(Suppl 1), 5–8.
Jeyaraman, N., Prajwal, G. S., Jeyaraman, M., Muthu, S., & Khanna, M. (2021). Chondrogenic potential of dental-derived mesenchymal stromal cells. Osteology, 1(3), 149–174. https://doi.org/10.3390/osteology1030016
Jeyaraman, M., Muthu, S., & Ganie, P. A. (2021). Does the source of mesenchymal stem cell have an effect in the management of osteoarthritis of the knee? Meta-analysis of randomized controlled trials. Cartilage, 13(1 Suppl), 1532S-1547S. https://doi.org/10.1177/1947603520951623
Zhu, C., Wu, W., & Qu, X. (2021). Mesenchymal stem cells in osteoarthritis therapy: A review. American Journal of Translational Research, 13(2), 448–461.
Muthu, S., Patil, S. C., Jeyaraman, N., Jeyaraman, M., Gangadaran, P., Rajendran, R. L., et al. (2023). Comparative effectiveness of adipose-derived mesenchymal stromal cells in the management of knee osteoarthritis: A meta-analysis. World Journal of Orthopedics, 14(1), 23–41. https://doi.org/10.5312/wjo.v14.i1.23
Muthu, S., Jeyaraman, M., Jain, R., Gulati, A., Jeyaraman, N., Prajwal, G. S., et al. (2021). Accentuating the sources of mesenchymal stem cells as cellular therapy for osteoarthritis knees—A panoramic review. Stem Cell Investigation, 8, 13. https://doi.org/10.21037/sci-2020-055
Jeyaraman, M., Verma, T., Jeyaraman, N., Patro, B. P., Nallakumarasamy, A., & Khanna, M. (2023). Is mandible derived mesenchymal stromal cells superior in proliferation and regeneration to long bone-derived mesenchymal stromal cells? World Journal of Methodology, 13(2), 10–17. https://doi.org/10.5662/wjm.v13.i2.10
Jeyaraman, M., Muthu, S., Gangadaran, P., Ranjan, R., Jeyaraman, N., Prajwal, G. S., et al. (2021). Osteogenic and chondrogenic potential of periosteum-derived mesenchymal stromal cells: Do they hold the key to the future? Pharmaceuticals, 14(11), 1133. https://doi.org/10.3390/ph14111133
Jeyaraman, M., Muthu, S., Jain, R., & Khanna, M. (2021). Autologous bone marrow derived mesenchymal stem cell therapy for osteonecrosis of femoral head: A systematic overview of overlapping meta-analyses. Journal of Clinical Orthopaedics and Trauma, 13, 134–142. https://doi.org/10.1016/j.jcot.2020.11.015
Lee, H.-S., Huang, G.-T., Chiang, H., Chiou, L.-L., Chen, M.-H., Hsieh, C.-H., et al. (2003). Multipotential mesenchymal stem cells from femoral bone marrow near the site of osteonecrosis. Stem Cells (Dayton, Ohio), 21(2), 190–199. https://doi.org/10.1634/stemcells.21-2-190
Li, C., Li, G., Liu, M., Zhou, T., & Zhou, H. (2016). Paracrine effect of inflammatory cytokine-activated bone marrow mesenchymal stem cells and its role in osteoblast function. Journal of Bioscience and Bioengineering, 121(2), 213–219. https://doi.org/10.1016/j.jbiosc.2015.05.017
Haumer, A., Bourgine, P. E., Occhetta, P., Born, G., Tasso, R., & Martin, I. (2018). Delivery of cellular factors to regulate bone healing. Advanced Drug Delivery Reviews, 129, 285–294. https://doi.org/10.1016/j.addr.2018.01.010
Hernigou, P., & Beaujean, F. (2002). Treatment of osteonecrosis with autologous bone marrow grafting. Clinical Orthopaedics and Related Research, 405, 14–23. https://doi.org/10.1097/00003086-200212000-00003
Wyles, C. C., Houdek, M. T., Crespo-Diaz, R. J., Norambuena, G. A., Stalboerger, P. G., Terzic, A., et al. (2015). Adipose-derived mesenchymal stem cells are phenotypically superior for regeneration in the setting of osteonecrosis of the femoral head. Clinical Orthopaedics and Related Research, 473(10), 3080–3090. https://doi.org/10.1007/s11999-015-4385-8
Oedayrajsingh-Varma, M. J., van Ham, S. M., Knippenberg, M., Helder, M. N., Klein-Nulend, J., Schouten, T. E., et al. (2006). Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy, 8(2), 166–177. https://doi.org/10.1080/14653240600621125
Sharma, S., Muthu, S., Jeyaraman, M., Ranjan, R., & Jha, S. K. (2021). Translational products of adipose tissue-derived mesenchymal stem cells: Bench to bedside applications. World Journal of Stem Cells, 13(10), 1360–1381. https://doi.org/10.4252/wjsc.v13.i10.1360
Wang, H.-J., Cai, B., Zhao, X.-Y., Li, S.-Q., Feng, W., Liu, J.-G., et al. (2017). Repairing diabetic rats with bone defect by VEGF165 gene modified adipose-derived stem cells. China Journal of Orthopaedics and Traumatology, 30(6), 545–551. https://doi.org/10.3969/j.issn.1003-0034.2017.06.012
Oswald, J., Boxberger, S., Jørgensen, B., Feldmann, S., Ehninger, G., Bornhäuser, M., et al. (2004). Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells (Dayton, Ohio), 22(3), 377–384. https://doi.org/10.1634/stemcells.22-3-377
Silva, G. V., Litovsky, S., Assad, J. A. R., Sousa, A. L. S., Martin, B. J., Vela, D., et al. (2005). Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation, 111(2), 150–156. https://doi.org/10.1161/01.CIR.0000151812.86142.45
Janeczek, P. K., Leferink, A., Groen, N., Fernandes, H., Moroni, L., van Blitterswijk, C., et al. (2012). Endothelial differentiation of mesenchymal stromal cells. PLoS ONE, 7(10), e46842. https://doi.org/10.1371/journal.pone.0046842
Zimmerlin, L., Donnenberg, V. S., Pfeifer, M. E., Meyer, E. M., Péault, B., Rubin, J. P., et al. (2010). Stromal vascular progenitors in adult human adipose tissue. Cytometry. Part A: The Journal of the International Society for Analytical Cytology, 77(1), 22–30. https://doi.org/10.1002/cyto.a.20813
Cao, Y. (2007). Angiogenesis modulates adipogenesis and obesity. The Journal of Clinical Investigation, 117(9), 2362–2368. https://doi.org/10.1172/JCI32239
Zuk, P. A., Zhu, M., Mizuno, H., Huang, J., Futrell, J. W., Katz, A. J., et al. (2001). Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Engineering, 7(2), 211–228. https://doi.org/10.1089/107632701300062859
Ramakrishnan, V. M., & Boyd, N. L. (2018). The adipose stromal vascular fraction as a complex cellular source for tissue engineering applications. Tissue Engineering. Part B, Reviews, 24(4), 289–299. https://doi.org/10.1089/ten.teb.2017.0061
Bora, P., & Majumdar, A. S. (2017). Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Research & Therapy, 8(1), 145. https://doi.org/10.1186/s13287-017-0598-y
Bourin, P., Bunnell, B. A., Casteilla, L., Dominici, M., Katz, A. J., March, K. L., et al. (2013). Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics (IFATS) and Science and the International Society for Cellular Therapy (ISCT). Cytotherapy, 15(6), 641–648. https://doi.org/10.1016/j.jcyt.2013.02.006
Klar, A. S., Güven, S., Zimoch, J., Zapiórkowska, N. A., Biedermann, T., Böttcher-Haberzeth, S., et al. (2016). Characterization of vasculogenic potential of human adipose-derived endothelial cells in a three-dimensional vascularized skin substitute. Pediatric Surgery International, 32(1), 17–27. https://doi.org/10.1007/s00383-015-3808-7
Morris, M. E., Beare, J. E., Reed, R. M., Dale, J. R., LeBlanc, A. J., Kaufman, C. L., et al. (2015). Systemically delivered adipose stromal vascular fraction cells disseminate to peripheral artery walls and reduce vasomotor tone through a CD11b+ cell-dependent mechanism. Stem Cells Translational Medicine, 4(4), 369–380. https://doi.org/10.5966/sctm.2014-0252
Aimaiti, A., Saiwulaiti, Y., Saiyiti, M., Wang, Y.-H., Cui, L., & Yusufu, A. (2011). Therapeutic effect of osteogenically induced adipose derived stem cells on vascular deprivation-induced osteonecrosis of the femoral head in rabbits. Chinese Journal of Traumatology, 14(4), 215–220.
Abudusaimi, A., Aihemaitijiang, Y., Wang, Y.-H., Cui, L., Maimaitiming, S., & Abulikemu, M. (2011). Adipose-derived stem cells enhance bone regeneration in vascular necrosis of the femoral head in the rabbit. The Journal of International Medical Research, 39(5), 1852–1860. https://doi.org/10.1177/147323001103900528
Pak, J. (2012). Autologous adipose tissue-derived stem cells induce persistent bone-like tissue in osteonecrotic femoral heads. Pain Physician, 15(1), 75–85.
Strioga, M., Viswanathan, S., Darinskas, A., Slaby, O., & Michalek, J. (2012). Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells and Development, 21(14), 2724–2752. https://doi.org/10.1089/scd.2011.0722
Lee, K., Kim, H., Kim, J.-M., Kim, J.-R., Kim, K.-J., Kim, Y.-J., et al. (2011). Systemic transplantation of human adipose-derived stem cells stimulates bone repair by promoting osteoblast and osteoclast function. Journal of Cellular and Molecular Medicine, 15(10), 2082–2094. https://doi.org/10.1111/j.1582-4934.2010.01230.x
Schäffler, A., & Büchler, C. (2007). Concise review: Adipose tissue-derived stromal cells–basic and clinical implications for novel cell-based therapies. Stem Cells (Dayton, Ohio), 25(4), 818–827. https://doi.org/10.1634/stemcells.2006-0589
Behr, B., Tang, C., Germann, G., Longaker, M. T., & Quarto, N. (2011). Locally applied vascular endothelial growth factor A increases the osteogenic healing capacity of human adipose-derived stem cells by promoting osteogenic and endothelial differentiation. Stem Cells (Dayton, Ohio), 29(2), 286–296. https://doi.org/10.1002/stem.581
Hua, K.-C., Yang, X.-G., Feng, J.-T., Wang, F., Yang, L., Zhang, H., et al. (2019). The efficacy and safety of core decompression for the treatment of femoral head necrosis: A systematic review and meta-analysis. Journal of Orthopaedic Surgery and Research, 14(1), 306. https://doi.org/10.1186/s13018-019-1359-7
Author information
Authors and Affiliations
Contributions
Conceptualization—VT, RS; Data procurement—MBP, AKS; Data analysis—RS; Manuscript writing—MJ, AN; Manuscript revision—MJ, AN; and Project administration—VT, RS. All authors agreed to publish the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed consent
For this type of study informed consent is not required.
IEC approval
IMCHRC/IEC/2015/013 dated 02.05.2015.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tantuway, V., Jeyaraman, M., Nallakumarasamy, A. et al. Functional Outcome Analysis of Autologous Stromal Vascular Fraction (SVF) (Sahaj Therapy®) Using Direct Sonication in Osteonecrosis of the Femoral Head (ONFH): A 6-Year Follow-Up Study. JOIO 58, 68–78 (2024). https://doi.org/10.1007/s43465-023-01041-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43465-023-01041-y