Abstract
Glycycoumarin is a representative coumarin compound with significant pharmacological activities isolated from Glycyrrhiza uralensis Fisch., Fabaceae. Studies have shown that glycycoumarin has many biological activities, such as anti-tumor, liver protection, antispasmodic, antibacterial, and antivirus. However, the poor solubility of glycycoumarin in water and the accompanying reactions of the phase I (hydroxylation) and II (glucuronidation) metabolism limit its druggability, which manifests as low absorption in the body after oral administration and low free drug concentration, ultimately leading to low bioavailability. Therefore, a comprehensive review of the pharmacological effects and pharmacokinetics of glycycoumarin is presented to provide a reference for further research and application as a therapeutic agent.
Graphical Abstract

Similar content being viewed by others
Introduction
Chinese licorice (Gan-Cao) is derived from the dried root or rhizome of Glycyrrhiza uralensis Fisch., Fabaceae, or its congeners G. inflata Batalin and G. glabra L. (Yang et al. 2019). Licorice is an ancient Chinese ethnomedicine used for invigorating the spleen and “qi” or vital energy; heat clearing and detoxifying; eliminating phlegm and relieving cough, spasm, and pain; and coordinating the drug actions of a prescription “Guo-Yao” in Chinese (Zeng et al. 1988; Liu et al. 2014). In modern pharmacological studies, licorice was confirmed to have various pharmacological effects including antiviral, immunomodulatory, cough expectorant, prevention of liver damage, antiarrhythmic, and corticosteroid-like effects (Zhang 2019). At present, licorice is widely used in medicine, cosmetics, food, and veterinary medicine worldwide, especially in the medical field for treatment of respiratory diseases, digestive diseases, hypotension, rheumatism, malaria, jaundice, bloating due to fluid retention, paralysis, sexual weakness, and certain viral infections (Batiha et al. 2020).
Licorice has numerous chemical constituents, including triterpenoids, flavonoids, coumarins, and alkaloids, as well as other natural active compounds, among which coumarin is one of the most important natural organic compounds (Hosseinzadeh and Nassiri-Asl 2015; Yin et al. 2018). Coumarin compounds in licorice have various structures, including simple coumarins, 3-arylcoumarins, coumarin-like, 4-arylcoumarins, and coumestans (Zang 2020). Zhu et al. (1984) first isolated glycycoumarin (1), which belongs to the 3-aryl-coumarin class (Song 2018). According to previous studies, 1 is mainly isolated from G. uralensis, with very low or undetectable levels in other species of licorice. Qiao et al. (2014c) collected different species of licorice in different provinces of China and found that the content of 1 was significantly higher in G. uralensis than in other species. Ye et al. (2014) compared the content of 1 and other components in various parts of licorice and found that this coumarin was not distributed in the stems, but it was found in leaves (0.061%), seeds (0.001%), seed coats (0.099%), and roots (0.0318–0.1741%).

It has been found that 1 has good pharmacological activity in vivo and can exert regulatory effects in the organism through various pathways, which mainly include anti-tumor (Li et al. 2019), protection against nonalcoholic and acetaminophen-induced liver injury (Zhang et al. 2016), antibacterial (Tanaka et al. 2001), antiviral (Sekine-Osajima et al. 2009; Adianti et al. 2014), anti-oxidation, anti-inflammatory (Fu et al. 2013), and antispasmodic (Lee et al. 2013), among the most important therapeutical effects. In vivo, it is metabolized mainly through hydroxylation and glucuronidation reactions by the cytochrome P450 and UDP-glucuronosyltransferase enzymatic system, respectively. Some studies have reported that 1 does not have significant cytotoxic effects at normal concentrations (Cheng et al. 2019), but causes cellular damage at concentrations higher than 5 mg/ml (Kasai et al. 2008). In this paper, the pharmacological activity and pharmacokinetics of 1 were summarized to provide reference for further pharmacological investigation of 1.
Search Strategies
A systematic literature search was performed in PubMed, Web of Science, China National Knowledge Infrastructure, Scopus, Embase, Google Scholar, and Sci-Finder Scholar databases. A search on glycycoumarin was done by using combinations of keywords, including “glycycoumarin,” “coumarin,” “Glycyrrhiza uralensis Fisch.,” “pharmacological activity,” “physicochemical properties,” “pharmacokinetics,” and “toxicity.” Considering the language limitation, this review only refers to Chinese and English texts. In the present study, the existing literature on the anti-hepatocellular cancer and liver damage protection effects of glycycoumarin (1) in an-liver disease drugs will be reviewed in addition to its possible mechanism.
Discussion
Physicochemical Properties
Glycycoumarin (1) is a lipophilic compound with strong lipid solubility with Log P > 3.2 (Wang et al. 2016a) and with a molecular weight, melting point, and density of 368.3799 g/mol, 243.5–244.5 °C/231 mmHg, and 1.3 ± 0.1 g/cm3, respectively. According to the study, 1 has good permeability and is transported in vivo mainly by passive diffusion (Wang et al. 2017), but its water solubility is poor and it is almost insoluble in water at room temperature as measured by the saturation shaking-ASK method (Ji et al. 2016). Also, on the chemspider database, the ACD/Labs Percepta Platform-PhysChem Module predicted oil–water partition coefficients of 5.99 (water), 4.38 (pH 5.5), and 4.25 (7.4); the water solubility estimate from Log Kow (WSKOW v1.41): water solubility at 25 ℃ is 4.11 mg/l, and the water solubility estimate from fragments is 3.8961 mg/l. It has been shown (Van Breemen et al. 2014) that the content of 1 in different varieties of licorice was determined by extraction by percolation, separation by the counter-current method, and UHPLC-MS/MS, where G. uralensis contained 0.13% (w/w) of 1, while G. inflata contained only 0.005% (w/w) of 1, which was not detected in G. glabra. It belongs to the drug type II class according to the BCS system classification in biopharmacology; its drug-forming properties are not ideal, which has a relevant effect on its pharmacokinetic properties; and its bioavailability in vivo after oral administration is not ideal.
Pharmacological Activity
Liver Damage Protection
Glycycoumarin (1) has protective effects against liver injury such as acetaminophen-induced acute liver injury, alcoholic liver disease, and non-alcoholic fatty liver disease. Multiple mechanisms have been identified to be involved in the protective effect of 1 on liver diseases, such as activating nuclear factor erythroid 2–related factor 2 (Nrf2) and autophagy, and inhibiting the T-lymphokine-activated killer cell–originated protein kinase (TOPK) and endoplasmic reticulum stress (Zhang et al. 2020a).
Acetaminophen-induced acute liver injury is a common cause of acute liver failure in developed countries, and N-acetyl cysteine is currently the only antidote approved by the FDA for the treatment of this hepatic injury (Polson and Lee 2005), but its therapeutic window is limited by the metabolic stage of N-acetyl-para-aminophenol (APAP), resulting in less than optimal efficacy (James et al. 2003). Yan et al. (2018) found that 1 was able to attenuate acetaminophen-induced mitochondrial oxidative stress and c-jun N-terminal kinase (JNK) pathway activation through sustained activation of autophagy, thereby avoiding liver injury. Endoplasmic reticulum phagocytosis, which accompanies endoplasmic reticulum stress, is activated when APAP is overdosed, and treatment with 1 enhances TEX264-mediated endoplasmic reticulum phagocytosis, thereby inhibiting endoplasmic reticulum stress and promoting liver regeneration, significantly reducing liver injury and, thus, its mortality (Yan et al. 2021). Glycycoumarin (1) is a potential replacement for N-acetyl cysteine as a therapeutic agent for patients with advanced acetaminophen-induced acute liver injury. Studies have found (Song et al. 2015) that 1 can effectively prevent alcohol-induced hepatic steatosis and can exert hepatoprotective effects in both chronic and acute alcoholic liver injury models. Glycycoumarin treatment activated Nrf2 at the transcriptional level and led to the upregulation of Nrf2 downstream target genes, heme oxygenase 1 (HO-1), and glutamate-cysteine ligase catalytic subunit, thereby reducing ethanol-induced cytotoxicity. At the same time, it leads to transcriptional upregulation of P62, which in turn promotes 1-induced activation of Nrf2 by inhibiting Keap1, thus forming a positive feedback loop of Nrf2 activation and exerting a protective effect. In addition, 1 also degraded P62-mediated Keap1 by activating autophagy, thereby reducing alcohol-induced hepatotoxicity.
Non-alcoholic fatty liver disease is a highly prevalent disease worldwide and is the more common type of liver disease. It has been shown that steatosis alone does not cause complications, but some patients develop severe liver damage such as steatohepatitis, liver fibrosis, and cirrhosis (Ratziu and Poynard 2010), which can lead to liver cancer in more severe cases. Zhang et al. (2016, 2017a, b) used a cell culture model of palmitate-induced hepatocyte apoptosis and a methionine/choline-deficient diet–induced non-alcoholic steatohepatitis mouse model to demonstrate that 1 protects against non-alcoholic fatty liver disease both in vitro and in vivo. In vitro, 1 significantly attenuated palmitic acid (PA)–mediated activation of JNK and C/EBP-homologous protein (CHOP); expression of the pro-apoptotic proteins Bax, Bak, PUMA, and Bim; and PA-reduced expression of the anti-apoptotic protein BCL2, thereby inhibiting adipocyte apoptosis. 1 inhibited phosphorylation of two substrates of m-TOR, S6K, and 4EBP1, and enhanced ULK1 phosphorylation and BTG1 expression to activate autophagy and play a preventive role against lipid accumulation. In vivo, GCM inhibits PA-mediated ER stress and autophagic response by attenuating the protein abundance of ER chaperone Bip, increased phosphorylation levels of PERK-EIF2α and IRE1α, and enhanced GSK-3 activation in the liver of NASH model mice, which contributes to the inhibition of JNK and CHOP activation. In addition, enhanced expression of the pro-apoptotic BCL2 family of proteins and decreased expression of the anti-apoptotic BCL2 family could be inhibited to suppress mitochondrial activation and regulate hepatic lipid metabolism.
Glycycoumarin (1) was also reported to activate adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK), enhance the inhibitory phosphorylation of acetyl CoA carboxylase (ACC), and enhance the activity of carnitine/palmitoyl-transferase 1A (CPT1A) to induce fatty acid oxidation. Glycycoumarin can increase the activation of PLIN5-Sirt1 axis in vitro and in vivo; regulate PA-induced endoplasmic reticulum stress and phosphorylation of IL-6, IRE1, and c-JNK; and exert protective effects against apoptosis, hepatocyte inflammation, and lipotoxicity (Fig. 1).
Anti-cancer
Anti-hepatocellular Carcinoma
China is a country with a high incidence of liver cancer, and the mortality rate is second only to lung cancer. Hepatocellular carcinoma can be divided into two categories: primary hepatocellular carcinoma refers to carcinomas that occur in hepatocytes or intrahepatic bile duct cells; secondary hepatocellular carcinoma (metastatic hepatocellular carcinoma) refers to malignant tumors of multiple organ origins throughout the body that invade the liver (Wang 2018). The incidence of primary hepatocellular carcinoma has been increasing worldwide in recent years, ranking 5th among malignant tumors and 3rd in mortality, and it is valuable to find new drug candidates to treat hepatocellular carcinoma. Song et al. (2016) first examined the anti-hepatocellular carcinoma activity of 1 in vitro and in vivo models and found that it activates P53 by directly inactivating TOPK, thereby causing cell cycle arrest, inducing cell death and tumor reduction in vivo. Glycycoumarin can exert a good anti-hepatoma effect in cell culture and the HepG2 xenograft model, and play a preventive effect on cancers induced by HepG2, Huh7, and human prostate cancer DU-145, and other human hepatoma cells (Hasan et al. 2021). The BH3 mimetic drug ABT-737 is a representative of good molecularly targeted therapeutic agents; Zhang et al. (2018) found that 1 significantly enhanced the antihepatocellular carcinoma efficacy of ABT-737 and reduced ABT-737–mediated platelet toxicity in both cell culture and tumor xenograft animal models. Its efficacy is enhanced mainly through two pathways: 1 inactivates the TOPK-survivin axis as well as inhibits de novo adipogenesis, while ABT-737 induces cell death by targeting anti-apoptotic BCL2 family proteins. Both pathways can be performed simultaneously; in addition, it did not have an enhanced effect on normal hepatocytes, which could avoid some adverse effects and has the potential to be a BH3-like drug sensitizer, facilitating the use of BH3-mimetic drugs as a new clinical option (Fig. 1).
Anti-bladder and Breast Cancer
Carcinogenesis is a multi-step process involving numerous genetic alterations, and single-drug intervention strategies often fail to lead to effective efficacy in clinical trials (Shaffer et al. 2012). The glycyrol/butyrate combination showed significant inhibition of HT29 and HCT116 cells, and it was found that glycycoumarin (1) alone did not show significant differences, but also showed significant inhibition when used in combination with butyrate, with a lower intensity of action than glycyrol/butyrate combination (Lu et al. 2020).
Bladder cancer is a malignant tumor occurring on the bladder mucosa. It is the ninth most common malignancy worldwide and a more common cause of cancer death (Dobruch et al. 2016). The incidence of bladder cancer is much higher in men than in women, with a global annual statistical report of 9.6/100,000 in men and 2.4/100,000 in women for 2019, and its incidence is positively correlated with increasing age (Richters et al. 2020). Yang et al. (2007) determined the effect of 11 coumarins including 1 on the proliferation of human bladder cancer cell line E-J by sulforhodamine B assay method, and it was found that the inhibition rate of E-J cell line proliferation increased slowly with the increase of 1 concentration and showed a concentration-effect-dependent relationship with an IC50 value of 1.90 × 10−5 mol/l. The inhibition rate was up to 90% when the 1 concentration was 10−4 mol/l, but the mechanism of action has not been reported yet and further studies are needed for follow-up.
During the evaluation of the activity of licorice components for estrogen-responsive gene expression regulation in MCF7 breast cancer cells, it was found that 1 was effective in increasing the expression of PgR and GREB1 and had intrinsic activity comparable to that of E2 ubiquitin-conjugating enzymes in the activation of estrogen-regulated genes and stimulating proliferation with significant agonist activity (Boonmuen et al. 2016). According to an in vitro activity assessment, 1 was found to have no anti-non-small-cell lung cancer activity (Lu et al. 2019).
Antibacterial
Minimum inhibitory concentration (MIC) is an index to measure the magnitude of antibacterial activity of antibacterial drugs and can be used to measure the ability of anti-infective drugs to resist pathogenic microorganisms, which is the lowest concentration that can inhibit the growth of bacteria in the culture medium. Demizu et al. (1988) studied the growth inhibition of various microorganisms, including bacteria, fungi, and yeast, by glycycoumarin (1), using MIC as a judgment indicator. The inhibitory effect of 1 on gram-positive bacteria was similar to that of streptomycin, where the inhibition of Staphylococcus aureus was extremely strong, and yeast, which was inactive against streptomycin, also showed significant activity, and showed inhibitory activity against fungi, such as Rhizopus formosaensis, but the inhibition of gram-negative bacteria was not obvious. Hee et al. (2011) studied the antifungal activity of 1 and found that it had a significant effect on Candida albicans, an opportunistic pathogenic yeast that is a common member of the human gut flora. The yeast form of C. albicans treated with 1 at 20 or 40 µg/ml resulted in 57% and 92% of hyphal production, respectively, as compared with the untreated control. Fungal growth was almost completely inhibited at a concentration of 320 µg/ml, comparable to the same dose of fluconazole. In addition, in a mouse model of disseminated candidiasis, the resistance of mice to disseminated disease was enhanced, and the protection of 1 against the disease was 60% during the observation period. Glycycoumarin (1) showed antibacterial activity against upper respiratory tract bacteria such as Streptococcus pyogenes ATCC 12,344 and Haemophilus influenzae ATCC 33,391 and Moraxella catarrhalis (Tanaka et al. 2001), Staphylococcus aureus ATCC 6538 (Kırmızıbekmez et al. 2015), and Enterococcus faecalis FN-1 (Eerdunbayaer et al. 2014), among others. The study found that the antibacterial activity of 1 against methicillin-resistant Staphylococcus aureus and methicillin-sensitive Staphylococcus aureus was higher than that of griseofulvin (Hatano et al. 2000), and the antibacterial activity was affected by the number of phenolic hydroxyl groups in the structure and the effect of methylation degree (Hatano et al. 2017). Combined with the results of 1 antimicrobial activity (Table S2), it can be speculated that this coumarin has the potential to develop antimicrobial active agents.
Antiviral
As a traditional Chinese herbal medicine, licorice itself has many pharmacological effects, among which antiviral effects have been confirmed. Glycycoumarin (1), glycyrrhizic acid, and glycyrol and other compounds isolated from licorice also have antiviral activity. Studies have reported their antiviral activity against hepatitis C virus (HCV), influenza virus, and human immunodeficiency virus (HIV), among others. It was found (Uchiumi et al. 2003) that 1 has antiviral effects against HIV, inhibits 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced anti-HIV promoter activity in Jurkat cells (Hatano et al. 2017), and has inhibitory effects on giant cell formation in HIV-infected cell lines. The study found that isoliquiritigenin and 1 isolated from licorice extracts specifically inhibited HCV replication in vitro, at different concentrations in culture with the Huh7/Rep-Feo cell line. Glycycoumarin (1) and isoliquiritigenin inhibited HCV replicon in a dose-dependent and time-dependent manner and were able to significantly inhibit intracellular HCV core protein expression with therapeutic index EC50 values of 15.5 ± 0.8 and 6.2 ± 1 mg/ml, respectively (Sekine-Osajima et al. 2009). The study found that compounds from Glycyrrhiza species could exert anti-HCV activity after cell infection. HCV was inoculated in Huh7.5 cells and then treated with 1, compared to the blank; the treated group was able to inhibit HCV RNA replication, and hepatitis virus protein was significantly reduced. 1 was able to inhibit the production of HCV infectious particles from 1 to 2 days of infection with an IC50 value of 8.8 µg/ml, and the cytotoxic CC50 was 69 µg/ml (Adianti et al. 2014; Wahyuni et al. 2014).
The 3C-like protease (3CLPro) is the main protease produced by the novel coronavirus (coronavirus disease 2019, COVID-19) (Bhati et al. 2021), and inhibition of 3CLpro can effectively inhibit virus infection and replication. The predicted results of the latest study showed (Abdizadeh et al. 2021) that 1 shows a better binding affinity to 3CLpro, with an optimal negative energy fraction, and interacts with one or two catalytic residues of 3CLpro (His41 and Cys145) through hydrophilic and hydrophobic bonds. The 3CLpro-GCM complex is highly stable with low conformational fluctuations and similar tightness. In addition, molecular kinetic simulations showed that the 3CLpro active site corresponding to 1 in COVID-19 was stable and had a significant binding free energy of − 60.31 kJ/mol, while pharmacokinetic and ADMET assessments also indicated their effectiveness as drug molecules. One study found (Abdelmohsen et al. 2021) that when screening coumarins to dock viral methyltransferases, among them, 1 docked with the best docking fractions of 9.2 kcal/mol. Therefore, 1 has the potential to be designed as an effective antiviral drug against COVID-19.
Antispasmodic
During the process of elucidating the antispasmodic effects of licorice by assaying its inhibitory effect on carbachol-induced jejunal contractions in mice, it was discovered that glycycoumarin (1) acts as an effective antispasmodic agent by inhibiting phosphodiesterase. In the process of exploring the contractile response of 1 on mouse jejunum, it was found that it had a significant inhibitory effect on the contraction of the jejunum, ileum, and colon induced by carbamylcholine. In addition, 1 was able to attenuate the sustained contractile response induced by carbachol, KCl, BaCl2, calcium carrier III, and acetylcholine, with IC50 values of 1.08 ± 0.35, 0.95 ± 0.29, 1.51 ± 0.67, and 2.72 ± 1.91 µg/ml, respectively. The anti-smooth muscle mechanism of action of 1 was similar to the inhibitory effect of the antispasmodic drug papaverine, which included inhibition of carbachol-induced contractions, showing a concentration-dependent manner to completely inhibit contractions induced by various stimulants, with an IC50 value of 2.93 ± 0.94 mmol/l. Inhibition of phosphodiesterase inhibitors, especially through intracellular accumulation of cAMP by isozyme 3, resulted in the inhibition of smooth muscle contractions induced by different types of stimulants, with an IC50 value of 0.11 ± 0.04 µmol/l (Sato et al. 2007). The study (Nagai et al. 2006) found that although 1 is one of the components of licorice extracts with a low content, it is an important bioactive chemical marker to G. uralensis. The relaxant activity of licorice and cultivated roots directly depends on their 1 content, and the relaxant effect is significantly and positively correlated with the 1 content. It was found that 1 inhibited intestinal tube contraction activity more strongly than isoliquiritigenin, and the IC50 of both was 3.6 × 10−6 and 2.7 × 10−6 mol/l for contraction induced by CCh (1 × 10−6 mol/l) and KCl (60 mmol/l) stimulation, respectively, and inhibited the intestinal tube contraction effect of trichothecene through cAMP phosphodiesterase, thereby inhibiting intestinal smooth muscle spasm. 1 exhibited calcium activating chloride channel inhibitory activity thereby decreasing the level of intestinal fluid secretion and relieving diarrhea in rats (Harada et al. 2021). The findings suggest that 1 is a very promising antispasmodic drug.
Antioxidant and Anti-inflammatory
Vitamin C, also known as ascorbic acid, is a highly effective antioxidant in the body and is commonly used to reduce the oxidative stress of the ascorbate peroxidase substrate. It was reported (Fu et al. 2013) that glycycoumarin (1) exhibited antioxidant activity, where ABTS ~ + free radical scavenging activity was higher than that of ascorbic acid, with an EC50 of 4.32 ± 0.13 µmol/l. It inhibited non-enzymatically induced lipid peroxidation in rat liver microsomes, demonstrating antioxidant activity with an EC50 of 11.9 ± 0.05 µmol/l. In addition, 1 significantly inhibited the secretion of prostaglandin E2 in a dose-dependent manner with an IC50 value of 30.5 ± 1.1 µmol/l. The inhibition rate reached more than 80% when 1 reached 10 µmol/l, and compared with indomethacin, 1 showed a higher inhibition rate of NO production (Wang et al. 2016b; Bai et al. 2020), demonstrating a good anti-inflammatory activity. Some studies reported that 1 is slightly superior to sulfonamide and antibiotics in terms of anti-inflammatory and anti-metamorphic activities (Yang et al. 2007), with subsequent in-depth studies on antioxidant and anti-inflammatory activities pending.
Neuroprotective Effects
Glycycoumarin (1) has a significant inhibitory effect on amyloid-β (Aβ) oligomer–induced neuronal death, significantly reducing and attenuating Aβ oligomer–induced activation of cysteine protease-3 and neuronal death at 10–50 mmol/l, producing a neuroprotective effect in a dose-dependent manner, but increasing neurotoxicity at concentrations up to 100 mmol/l (Kanno et al. 2015; Ikarashi and Mizoguchi 2016; Batiha et al. 2020). And the activity of 1 for the treatment of Alzheimer’s disease needs to be further explored.
Anorexia Nervosa
Glycycoumarin (1) effectively inhibited the serotonin receptors 5-HT2B receptor, 5-HT2C receptor, and 5-HT4 receptor, binding with Ki values of 2.4 ± 1.4, 8.2 ± 1.8, and 4.1 ± 0.5 µmol/l, respectively, and inhibited anorexia and enhanced acyl growth hormone by inhibiting the reduction of food intake after 3 h of novel stress after oral administration (Takeda et al. 2008; Saegusa et al. 2011; Hattori 2016). In addition, studies have shown that 1 has antagonist effects with the α2-adrenergic receptor (Takeda et al. 2012), with IC50 values of 5.2 ± 0.7, 39.4 ± 3.3, and 14.5 ± 0.3 µmol/l for transfected cells of human AR subtypes, α2-ARA, α2-ARB, and α2-ARC, respectively (Yakabi et al. 2014), and has potential to be developed as a 5-HT receptor antagonist for the prevention and treatment of vomiting.
Cardioprotective Effects
Cyclic adenosine monophosphate (cAMP) phosphodiesterases are enzymes that catabolize cyclic adenosine acids under the activation of calmodulin bound to Ca2+. cAMP phosphodiesterase inhibitors inhibit type III phosphodiesterase activity in cardiac muscle and vascular smooth muscle cells, increasing intracellular cAMP content, which enhances myocardial contraction, dilates peripheral blood vessels, and improves hemodynamics in patients with heart failure. Compounds with inhibitory effects on cAMP phosphodiesterase activity have potential cardiotonic effects and can be developed as cardiotonic agents. 1, as an ingredient in Tongmai Yangxin pills, has been reported in a study (Tao et al. 2015) to possess myocardial protective activity. In exploring the effects of compounds in licorice root on cAMP phosphodiesterase activity (Kusano et al. 1991), glycycoumarin (1) was found to significantly inhibit the activity of this enzyme and has a potential cardiac-strengthening effect.
Other Effects
Glycycoumarin (1) directly increases glutathione levels without inducing xCT or 4F2hc gene expression to protect against glutamate-induced neuronal cell or PC12 cell death showing potent cytoprotective effects (Mizoguchi and Ikarashi 2017). Glycycoumarin (1) has a strong competitive inhibitory effect on butyrylcholinesterase (BuChE) activity and can act as a competitive inhibitor of BuChE (Sadakane et al. 2011). In addition, 1 exhibits a weak inhibitory activity against PD-1/PD-L1 (Bao et al. 2021), and can slow down the formation of gout by inhibiting xanthine oxidase activity (Mali and Joshi 2006). Glycycoumarin (1) promotes the proliferation and angiogenesis of zebrafish umbilical vein endothelial cells (Shen et al. 2018) and inhibits glutamate-induced neuronal and PC12 cell death (Ji and Kawakami 2013), among other effects.
Studies have reported that carboxylesterase (CES) inhibitors can ameliorate the intestinal toxicity of CES2A substrates (Fukami et al. 2015). Licorice has a strong inhibitory effect on CES2A and a relatively weak inhibitory effect on CES1A. further studies have found that GCM has a moderate inhibitory effect on CES2A in human liver microsomes with an IC50 value of 6.75 ± 0.88 µmol/l (Song et al. 2021), contributing to the development of novel CES2A inhibitors to reduce hCES2A-related drug toxicity. It has been reported that (Sanechika et al. 2021) 1 is effective in alleviating poor circulation via enhancement of TRPA1 induced CGRP release, and cold allodynia, migraine head-ache (Gavva et al. 2019), menopausal vasomotor symptoms and bladder pain (Mukerji et al. 2006) via TRPM8 inhibition, and with up to 97% inhibition of 5.4 nmol/l icilin and an IC value of 3.5 ± 0.4 μmol/l.
Acute lung injury (ALI) is damage to alveolar epithelial cells and capillary endothelial cells caused by various direct and indirect injury–causing factors (Wang and Tang 2021), and pulmonary edema and inflammatory cell infiltration of lung tissue due to pulmonary vascular injury are pathological features (Yasmeen et al. 2016). Patients clinically diagnosed with ALI present chest pain, chest tightness, and shortness of breath (Lin 2021). One study found (Ren 2016) that the traditional medicine prescription Dayuanyin made up of the seeds of Areca catechu L., Arecaceae; the cortex of Magnolia officinalis Rehder & E.H.Wilson, Magnoliaceae; the fruits of black cardamom, Amomum subulatum Roxb., Zingiberaceae; the rhizoma of Anemarrhena asphodeloides Bunge, Asparagaceae; the roots of Scutellaria baicalensis Georgi, Lamiaceae; the root of Paeonia lactiflora Pall., Paeoniaceae; and the root of G. uralensis has a therapeutic effect on lipopolysaccharide-induced acute lung injury in mice, which was associated with the reduction of pro-inflammatory factors, upregulation of the content of anti-inflammatory factors, and reduction of complement levels. It was identified that Dayuanyin contained various components such as 1, isoliquiritigenin, and glycyrol, from which it can be speculated that 1 may have protective effects against acute lung injury in mice, but further confirmation is needed.
Pharmacokinetics
A recent study reported (Kuang et al. 2021) that glycycoumarin (1) can be used as a UGT1A9 inhibitor with glabrone as a probe substrate, and when combined with the oral hypoglycemic drug class dapagliflozin, plasma concentrations of dagliflozin-O-glucuronide were found to be significantly reduced, while plasma free dapagliflozin was significantly increased after administration. Therefore, there may be a risk of drug interactions when 1 is used with drugs that require UDP-glucuronosyltransferase 1–9 (UGT1A9) metabolism, such as hypoglycemic agents during drug administration. In addition, some studies have found (Ko et al. 2007; Gou et al. 2016; Fan et al. 2020) that 1 has inhibitory effects on α-glucosidase, perhaps related to the antidiabetic effects of licorice, one of the pathways of which may be the activation of peroxisome proliferator-activated receptor gamma (PPAR-γ), also known as as the glitazone reverse insulin resistance receptor. Glycycoumarin (1) can inhibit cytochrome P450 (CYP) enzyme systems, and it has been shown that 1 can inhibit CYP1A2, CYP2B6, CYP2C, and CYP2C9 (Van Breemen et al. 2014) multiple CYP enzyme systems. In addition, 1 significantly inhibits CYP2D6 activity, which mediates the metabolism of approximately 30% of the drugs on the market, and is one of the components in licorice extracts that play a major role in inhibiting the overall activity of CYP450 enzyme systems (Qiao et al. 2014b). However, because of the low concentration of 1 in licorice extracts, licochalcone A remains a significant contributor to CYP enzyme inhibition. In addition, glycycoumarin has inhibitory activity against growth hormone–releasing peptide deacylase and butyrylcholinesterase (Mogami and Hattori 2014). Therefore, drug interactions may occur when 1 is co-administered with drugs metabolized by these aforementioned enzymes.
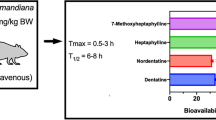
It was reported that the bioavailability of orally purified 1 in rats was only 13.82%, which was rapidly converted into conjugates after entering the body. Only a small portion was excreted through bile and urine, its half-life was short, and its Cmax appeared at 0.79 ± 0.64 h. Almost no free drug was detected in plasma 2 h after administration; in addition, the drug was widely distributed in tissues after administration, with a predominance in the liver, but did not cross the blood–brain barrier, showing potential liver targeting. Wang et al. (2014) used the HPLC–MS-coupled technique as well as NMR to identify the metabolites after oral administration of 1 to rats. The results of the study revealed that 1 was orally absorbed into the body circulation, where it was still mainly present in plasma and urine in unchanged form, and its main metabolic reactions in vivo were hydroxylation (phase I metabolites: M1, M2, and M9) and glucuronidation (phase II metabolites: M6, M7, and M8) reactions, followed by a small proportion of dehydrogenation (M3) and hydrogenation (M4 and M5) metabolic products (Fig. 2). Ji et al. (2016) first found that the isopentenyl O-hydroxyl group present on 1 can effectively undergo glycosylation reactions, and 7-O-glucoside is the main product. Combined with the pharmacokinetic studies, it is evident that 1 is poorly absorbed in vivo, and strategies to improve its absorption need to be further explored.
Qiao et al. (2012) compared the pharmacokinetic differences between multicomponent herbs and single compounds and the metabolic interactions between compounds by LC–MS-MS assay of licorice extract and single compounds in rats and found that the absorption and distribution of 1 were faster in rats with Tmax and t1/2 less than 1 h and 4 h, respectively, while the Cmax of glycyrrhetinic acid (GA) appeared at 13.6 ± 5.72 h. It was found that there were significant differences in pharmacokinetic parameters between licorice extracts and single compounds, where the area under the curve (AUC) of 1 increased and t1/2 was prolonged. The findings suggest that the multi-component system of licorice can improve the bioavailability of the compounds in vivo. Sadakane et al. (2015) determined the blood concentration of each component by HPLC–MS/MS in healthy adult volunteers after oral administration of peony licorice soup, in which 1 was 118 µg/g and GA was 5.29 µg/g in the soup, and found that the maximum blood concentration of GA after the soup was higher than that of 1. The concentration of 1 in plasma showed a bimodal curve, in which 1 was absorbed rapidly after administration. The maximum blood concentration of 1 was reached at 30 min, and the second concentration peak appeared at 2–3 h of administration, but the bimodal phenomenon did not appear in animals when administered intravenously (Qiao et al. 2014a), thus excluding the enterohepatic circulation. The study found that the herbal combination was able to improve the permeability of compounds in Ge Gen Scutellaria soup, which increased the transport of 1 by 30%, and the herbal combination was able to significantly alter the intestinal transport of the compounds (Wang et al. 2017). It is hypothesized that licorice as a multi-component system, when comparing the in vivo pharmacokinetic differences with each component alone, is able to alter the pharmacokinetic behavior of its compounds in different ways (Qiao et al. 2014b). Combined with the pharmacokinetic differences of the herbal extracts, it was found that the pharmacokinetics of the isolated compounds were greatly influenced when the herbs were combined.
Perspectives and Future Directions
Licorice is known as a herbal compounding and blending agent with many valuable and important pharmacological activities, mainly because it is a combination of several natural active compounds and therefore has a good clinical effect and is widely used (Yang et al. 2015). Glycycoumarin (1) is a representative coumarin-like compound in licorice with many potential pharmacological activities. Current anti-cancer strategies are not ideal, and the current situation of high cancer morbidity and mortality requires researchers to investigate preventive methods and alternative therapies (Zhang et al. 2017b). Glycycoumarin plays an active role in human cancers, such as liver, breast, bladder, and colon cancers. In this paper, the mechanism of action of GCM with anti-hepatocellular carcinoma and protection against liver injury in vitro and in vivo was summarized and confirmed the good activity of 1 in the treatment of hepatocellular carcinoma and protection against liver injury. In addition, 1 has many activities such as antibacterial, antiviral, anti-inflammatory, and antioxidant, as well as neuroprotective and cardioprotective, which have the potential to promote the future of human medicine. However, there are gaps in the pharmacological screening of 1. Specifically, the in vivo activities need to be further investigated.
The metabolism of 1 in vivo is complicated, and a variety of enzymes such as CYP450 enzymes are involved in its metabolic activation process, which may lead to drug interactions and toxic effects in vivo when combined with drugs. According to the study reports, it can be speculated that the low bioavailability of 1 may be related to its low solubility, hepatic first-pass effect, and phase II reaction, which affects the absorption into the body and reduces the drug efficacy.
For these reasons, as a lead compound with developmental value, improving the solubility of 1 as well as enhancing its bioavailability is a research gap that needs to be filled in the future. Currently, common methods to improve drug solubility and bioavailability include structural modification of compounds and pharmacological methods (Wang 2016), e.g., cyclodextrin encapsulation, addition of solubilizers or co-solvents and latent solvents, and preparation of solid dispersions, nanocrystals, microemulsions, and liposomes, among others, as well as the change of drug delivery routes. It has been reported that the structure of 1 as part of a 7-O-β-D-glucoside was able to significantly improve water solubility, promotes intestinal absorption, and increases drug-forming properties (Ji et al. 2016). In addition, the glycosylated compounds exhibited good Nrf2 activation activity (Liang et al. 2015), which shows that changing the structure of the compounds is a way to improve the solubility of 1. Since 1 belongs to the BCS II class of drugs, the absorption of the drug depends on the solubility, and its pharmacokinetics are easily affected by the particle size, dissolution rate, or excipients. To improve the bioavailability of BCS II drugs and increase their solubility and formulation dissolution thus improving oral absorption, the following methods are usually adopted: making soluble salts and amorphous drugs, adding appropriate amounts of surfactants, making inclusion compounds from hydrophilic inclusion materials, increasing the retention time of the drug in the gastrointestinal tract, increasing the specific surface area of the drug, and inhibiting the efflux transport and metabolism of the drug in the intestinal wall (Cao et al. 2021).
Conclusion
Although licorice is a relatively traditional Chinese herbal medicine, glycycoumarin (1), as one of its representative coumarins, is currently in a blank stage in the field of chemical synthesis as well as a drug formulation in pharmaceutical technology. The studies summarized in this review confirm the potential of 1 for drug development. Further systematic and comprehensive studies on the chemical synthesis, pharmacological effects, and in vivo metabolic processes of 1 should be carried out to promote the development of this bioactive coumarin as a new lead drug for clinical use.
Data Availability
The data that supports the findings of this study are available in the supplementary material of this article.
Change history
06 October 2023
A Correction to this paper has been published: https://doi.org/10.1007/s43450-023-00456-w
References
Abdelmohsen UR, Albohy A, Abdulrazik BS, Bayoumi SA, Malak LG, Khallaf IS, Bringmann G, Farag SF (2021) Natural coumarins as potential anti-SARS-CoV-2 agents supported by docking analysis. RSC Adv 11:16970–16979. https://doi.org/10.1039/d1ra01989a
Abdizadeh R, Hadizadeh F, Abdizadeh T (2021) In silico analysis and identification of antiviral coumarin derivatives against 3-chymotrypsin-like main protease of the novel coronavirus SARS-CoV-2. Mol Divers 26:1053–1076. https://doi.org/10.1007/s11030-021-10230-6
Adianti M, Aoki C, Komoto M, Deng L, Shoji I, Wahyuni TS, Lusida MI, Soetjipto FH, Kawahara N, Hotta H (2014) Anti-hepatitis C virus compounds obtained from Glycyrrhiza uralensis and other Glycyrrhiza species. Microbiol Immunol 58:180–187. https://doi.org/10.1111/1348-0421.12127
Bai H, Bao F, Fan X, Han S, Zheng W, Sun L, Yan N, Du H, Zhao H, Yang Z (2020) Metabolomics study of different parts of licorice from different geographical origins and their anti-inflammatory activities. J Sep Sci 43:1593–1602. https://doi.org/10.1002/jssc.201901013
Bao F, Bai HY, Wu ZR, Yang ZG (2021) Phenolic compounds from cultivated Glycyrrhiza uralensis and their PD-1/PD-L1 inhibitory activities. Nat Prod Res 35:562–569. https://doi.org/10.1080/14786419.2019.1586698
Batiha GE-S, Beshbishy AM, El-Mleeh A, Abdel-Daim MM, Devkota HP (2020) Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 10:352. https://doi.org/10.3390/biom10030352
Bhati S, Kaushik V, Singh J (2021) Rational design of flavonoid based potential inhibitors targeting SARS-CoV 3CL protease for the treatment of COVID-19. J Mol Struct 1237:130380. https://doi.org/10.1016/j.molstruc.2021.130380
Boonmuen N, Gong P, Ali Z, Chittiboyina AG, Khan I, Doerge DR, Helferich WG, Carlson KE, Martin T, Piyachaturawat P, Katzenellenbogen JA, Katzenellenbogen BS (2016) Licorice root components in dietary supplements are selective estrogen receptor modulators with a spectrum of estrogenic and anti-estrogenic activities. Steroids 105:42–49. https://doi.org/10.1016/j.steroids.2015.11.006
Cao QL, Han XL, Gao J, Zheng AP, Hu CD (2021) Research progress on improving bioavailability of insoluble drugs. J Hubei Univer Sci Technol 35:352–356. https://doi.org/10.16751/j.cnki.2095-4646.2021.04.0352
Cheng M, Ding LQ, Kan HF, Zhang HM, Jiang BK, Sun YJ, Cao SJ, Li W, Koike K, Qiu F (2019) Isolation, structural elucidation and in vitro hepatoprotective activity of flavonoids from Glycyrrhiza uralensis. J Nat Med 73:847–854. https://doi.org/10.1007/s11418-019-01329-0
Demizu S, Kajiyama K, Takahashi K, Hiraga Y, Yamamoto S, Tamura Y, Okada K, Kinoshita T (1988) Antioxidant and antimicrobial constituents of licorice: isolation and structure elucidation of a new benzofuran derivative. Chem Pharm Bull 36:3474–3479. https://doi.org/10.1248/cpb.36.3474
Dobruch J, Daneshmand S, Fisch M, Lotan Y, Noon AP, Resnick MJ, Shariat SF, Zlotta AR, Boorjian SA (2016) Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur Urol 69:300–310. https://doi.org/10.1016/j.eururo.2015.08.037
Eerdunbayaer OMA, Aoyama H, Kuroda T, Hatano T (2014) Structures of new phenolics isolated from licorice, and the effectiveness of licorice phenolics on vancomycin-resistant Enterococci. Molecules 19:13027–13041. https://doi.org/10.3390/molecules190913027
Fan JR, Kuang Y, Dong ZY, Yi Y, Zhou YX, Li B, Qiao X, Ye M (2020) Prenylated phenolic compounds from the aerial parts of Glycyrrhiza uralensis as PTP1B and alpha-glucosidase inhibitors. J Nat Prod 83:814–824. https://doi.org/10.1021/acs.jnatprod.9b00262
Fu Y, Chen J, Li YJ, Zheng YF, Li P (2013) Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem 141:1063–1071. https://doi.org/10.1016/j.foodchem.2013.03.089
Fukami T, Kariya M, Kurokawa T, Iida A, Nakajima M (2015) Comparison of substrate specificity among human arylacetamide deacetylase and carboxylesterases. Eur J Pharm Sci 78:47–53. https://doi.org/10.1016/j.ejps.2015.07.006
Gavva NR, Sandrock R, Arnold GE, Davis M, Lamas E, Lindvay C, Li CM, Smith B, Backonja M, Gabriel K, Vargas G (2019) Reduced TRPM8 expression underpins reduced migraine risk and attenuated cold pain sensation in humans. Sci Rep 9:19655. https://doi.org/10.1038/s41598-019-56295-0
Gou SH, Liu J, He M, Qiang Y, Ni JM (2016) Quantification and bio-assay of α-glucosidase inhibitors from the roots of Glycyrrhiza uralensis Fisch. Nat Prod Res 30:2130–2134. https://doi.org/10.1080/14786419.2015.1114940
Harada Y, Sekine H, Kubota K, Sadatomi D, Iizuka S, Fujitsuka N (2021) Calcium-activated chloride channel is involved in the onset of diarrhea triggered by EGFR tyrosine kinase inhibitor treatment in rats. Biomed Pharmacother 141:111860. https://doi.org/10.1016/j.biopha.2021.111860
Hasan MK, Ara I, Mondal MSA, Kabir Y (2021) Phytochemistry, pharmacological activity, and potential health benefits of Glycyrrhiza glabra. Heliyon 7:e07240. https://doi.org/10.1016/j.heliyon.2021.e07240
Hatano T, Eerdunbayaer, Cui YM, Kuroda T, Shimozu Y (2017) Licorice as a resource for pharmacologically active phenolic substances: antioxidant and antimicrobial effects. In: Sakagami H (ed). Biological Activities and Action Mechanisms of Licorice Ingredients. London: IntechOpen. https://doi.org/10.5772/66419
Hatano T, Shintani Y, Aga Y, Shiota S, Tsuchiya T, Yoshida T (2000) Phenolic constituents of licorice. VIII. Structures of glicophenone and glicoisoflavanone, and effects of licorice phenolics on methicillin-resistant Staphylococcus aureus. Chem Pharm Bull 48:1286–1292. https://doi.org/10.1248/cpb.48.1286
Hattori T (2016) Rikkunshito and Ghrelin Int J Pept 2010:283549. https://doi.org/10.1155/2010/283549
Hosseinzadeh H, Nassiri-Asl M (2015) Pharmacological effects of Glycyrrhiza spp. and its bioactive constituents: update and review. Phytother Res 29:1868–1886. https://doi.org/10.1002/ptr.5487
Ikarashi Y, Mizoguchi K (2016) Neuropharmacological efficacy of the traditional Japanese Kampo medicine yokukansan and its active ingredients. Pharmacol Ther 166:84–95. https://doi.org/10.1016/j.pharmthera.2016.06.018
James LP, McCullough SS, Lamps LW, Hinson JA (2003) Effect of N-acetylcysteine on acetaminophen toxicity in mice: relationship to reactive nitrogen and cytokine formation. Toxicol Sci 75:458–467. https://doi.org/10.1093/toxsci/kfg181
Ji S, Liang WF, Li ZW, Feng J, Wang Q, Qiao X, Ye M (2016) Efficient and selective glucosylation of prenylated phenolic compounds by Mucor hiemalis. RSC Adv 6:20791–20799. https://doi.org/10.1039/C6RA00072J
Kanno H, Kawakami Z, Tabuchi M, Mizoguchi K, Ikarashi Y, Kase Y (2015) Protective effects of glycycoumarin and procyanidin B1, active components of traditional Japanese medicine yokukansan, on amyloid β oligomer-induced neuronal death. J Ethnopharmacol 159:122–128. https://doi.org/10.1016/j.jep.2014.10.058
Kasai A, Hiramatsu N, Hayakawa K, Yao J, Kitmura M (2008) Blockade of the dioxin pathway by herbal medicine Formula Bupleuri Minor: identification of active entities for suppression of AhR activation. Biol Pharm Bull 31:838–846. https://doi.org/10.1248/bpb.31.838
Kırmızıbekmez H, Uysal GB, Masullo M, Demirci F, Bağcı Y, Kan Y, Piacente S (2015) Prenylated polyphenolic compounds from Glycyrrhiza iconica and their antimicrobial and antioxidant activities. Fitoterapia 103:289–293. https://doi.org/10.1016/j.fitote.2015.05.003
Ko BS, Jang JS, Hong SM, Sung SR, Lee JE, Lee MY, Jeon WK, Park S (2007) Changes in components, glycyrrhizin and glycyrrhetinic acid, in raw Glycyrrhiza uralensis Fisch, modify insulin sensitizing and insulinotropic actions. Biosci Biotechnol Biochem 71:1452–1461. https://doi.org/10.1271/bbb.60533
Kuang Y, Chai Y, Xu L, Wang Z, Liang L, Qiao X, Ye M (2021) Glabrone as a specific UGT1A9 probe substrate and its application in discovering the inhibitor glycycoumarin. Eur J Pharm Sci 161:105786. https://doi.org/10.1016/j.ejps.2021.105786
Kusano A, Nikaido T, Kuge T, Ohmoto T, Delle Monache G, Botta B, Botta M, Saitoh T (1991) Inhibition of adenosine 3’,5’-cyclic monophosphate phosphodiesterase by flavonoids from licorice roots and 4-arylcoumarins. Chem Pharm Bull 39:930–933. https://doi.org/10.1248/cpb.39.930
Lee JH, Lee YM, Han Y (2011) Antifungal activity of glycycoumarin to Candida albicans. Yakhak Hoeji 55:234–239
Lee KK, Omiya Y, Yuzurihara M, Kase Y, Kobayashi H (2013) Antispasmodic effect of shakuyakukanzoto extract on experimental muscle cramps in vivo: role of the active constituents of Glycyrrhizae radix. J Ethnopharmacol 145:286–293. https://doi.org/10.1016/j.jep.2012.11.005
Li X, Sun R, Liu R (2019) Natural products in licorice for the therapy of liver diseases: progress and future opportunities. Pharmacol Res 144:210–226. https://doi.org/10.1016/j.phrs.2019.04.025
Liang WF, Li ZW, Ji S, Qi W, Min Y (2015) Microbial glycosylation of tanshinone IIA by Cunninghamella elegans AS 3.2028. RSC Adv 5:63753–63756
Lin NJ (2021) Clinical effect of glucocorticoid therapy in children with acute lung injury. Chinese J Clin Rational Drug Use 14:129–131. https://doi.org/10.15887/j.cnki.13-1389/r.2021.35.052
Liu Y, Chen HH, Wen H, Gao Y, Wang LQ, Liu CS (2014) Enhancing the accumulation of beta-amyrin in Saccharomyces cerevisiae by co-expression of Glycyrrhiza uralensis squalene synthase 1 and beta-amyrin synthase genes. Yao Xue Xue Bao 49:734–741
Lu S, Ye L, Yin S, Zhao C, Yan M, Liu X, Cui J, Hu H (2019) Glycyrol exerts potent therapeutic effect on lung cancer via directly inactivating T-LAK cell-originated protein kinase. Pharmacol Res 147:104366. https://doi.org/10.1016/j.phrs.2019.104366
Lu S, Yin S, Zhao C, Fan L, Hu H (2020) Synergistic anti-colon cancer effect of glycyrol and butyrate is associated with the enhanced activation of caspase-3 and structural features of glycyrol. Food Chem Toxicol 136:110952. https://doi.org/10.1016/j.fct.2019.110952
Mali RS, Joshi PP (2006) Useful syntheses of prenylated- and pyrano-3-arylcoumarins. Synthetic Commun 31:2753–2760
Mizoguchi K, Ikarashi Y (2017) Cellular pharmacological effects of the traditional Japanese kampo medicine Yokukansan on brain cells. Front Pharmacol 8:655. https://doi.org/10.3389/fphar.2017.00655
Mogami S, Hattori T (2014) Beneficial effects of rikkunshito, a Japanese kampo medicine, on gastrointestinal dysfunction and anorexia in combination with Western drug: a systematic review. Evid Based Complement Alternat Med 2014:519035. https://doi.org/10.1155/2014/519035
Mukerji G, Yiangou Y, Corcoran SL, Selmer IS, Smith GD, Benham CD, Bountra C, Agarwal SK, Anand P (2006) Cool and menthol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urol 6:6. https://doi.org/10.1186/1471-2490-6-6
Nagai H, Yamamoto Y, Sato Y, Akao T, Tani T (2006) Pharmaceutical evaluation of cultivated Glycyrrhiza uralensis roots in comparison of their antispasmodic activity and glycycoumarin contents with those of licorice. Biol Pharm Bull 29:2442–2445. https://doi.org/10.1248/bpb.29.2442
Polson J, Lee WM (2005) AASLD position paper: The management of acute liver failure. Hepatology 41:1179–1197. https://doi.org/10.1002/hep.20703
Qiao X, Ye M, Xiang C, Wang Q, Liu CF, Miao WJ, Guo DA (2012) Analytical strategy to reveal the in vivo process of multi-component herbal medicine: a pharmacokinetic study of licorice using liquid chromatography coupled with triple quadrupole mass spectrometry. J Chromatogr A 1258:84–93. https://doi.org/10.1016/j.chroma.2012.08.041
Qiao H, Zhang XY, Wang T, Liang L, Chang W, Xia HX (2014a) Pharmacokinetics, biodistribution and bioavailability of isoliquiritigenin after intravenous and oral administration. Pharm Biol 52:228–236. https://doi.org/10.3109/13880209.2013.832334
Qiao X, Ji S, Yu SW, Lin XH, Jin HW, Duan YK, Zhang LR, Guo DA, Ye M (2014b) Identification of key licorice constituents which interact with cytochrome P450: evaluation by LC/MS/MS cocktail assay and metabolic profiling. AAPS J 16:101–113. https://doi.org/10.1208/s12248-013-9544-9
Qiao X, Liu CF, Ji S, Lin XH, Guo DA, Ye M (2014c) Simultaneous determination of five minor coumarins and flavonoids in Glycyrrhiza uralensis by solid-phase extraction and high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Planta Med 80:237–242. https://doi.org/10.1055/s-0033-1360272
Ratziu V, Poynard T (2010) Assessing the outcome of nonalcoholic steatohepatitis? It’s time to get serious. Hepatology 44:802–805. https://doi.org/10.1002/hep.21391
Ren HL (2016) The therapeutic effect of Dayuanyin on lipopolysaccharide -induced acute lung injury in mice. Soochow University. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201701&filename=1016225927.nh
Richters A, Aben KKH, Kiemeney L (2020) The global burden of urinary bladder cancer: an update. World J Urol 38:1895–1904. https://doi.org/10.1007/s00345-019-02984-4
Sadakane C, Muto S, Nakagawa K, Ohnishi S, Saegusa Y, Nahata M, Hattori T, Asaka M, Takeda H (2011) 10-Gingerol, a component of rikkunshito, improves cisplatin-induced anorexia by inhibiting acylated ghrelin degradation. Biochem Biophy Res Commun 412:506–511. https://doi.org/10.1016/j.bbrc.2011.08.002
Sadakane C, Watanabe J, Fukutake M, Nisimura H, Maemura K, Kase Y, Kono T (2015) Pharmacokinetic profiles of active components after oral administration of a kampo medicine, Shakuyakukanzoto, to healthy adult Japanese volunteers. J Pharm Sci 104:3952–3959. https://doi.org/10.1002/jps.24596
Saegusa Y, Takeda H, Muto S, Nakagawa K, Ohnishi S, Sadakane C, Nahata M, Hattori T, Asaka M (2011) Decreased plasma ghrelin contributes to anorexia following novelty stress. Am J Physiol Endocrinol Metab 301:E685–E696. https://doi.org/10.1152/ajpendo.00121.2011
Sanechika S, Shimobori C, Ohbuchi K (2021) Identification of herbal components as TRPA1 agonists and TRPM8 antagonists. J Nat Med 75:717–725. https://doi.org/10.1007/s11418-021-01515-z
Sato YJ, He JX, Nagai H, Tani T, Akao T (2007) Isoliquiritigenin, one of the antispasmodic principles of Glycyrrhiza ularensis roots, acts in the lower part of intestine. Biol Pharm Bull 30:145–149. https://doi.org/10.1248/bpb.30.145
Sekine-Osajima Y, Sakamoto N, Nakagawa M, Itsui Y, Tasaka M, Nishimura-Sakurai Y, Chen CH, Suda G, Mishima K, Onuki Y, Yamamoto M, Maekawa S, Enomoto N, Kanai T, Tsuchiya K, Watanabe M (2009) Two flavonoids extracts from Glycyrrhizae radix inhibit in vitro hepatitis C virus replication. Hepatol Res 39:60–69. https://doi.org/10.1111/j.1872-034x.2008.00398.x
Shaffer BC, Gillet JP, Patel C, Baer MR, Bates SE, Gottesman MM (2012) Drug resistance: still a daunting challenge to the successful treatment of AML. Drug Resist Updat 15:62–69. https://doi.org/10.1016/j.drup.2012.02.001
Shen JY, Wei J, Li L, OuYang HZ, Chang YX, Chen XP, He J (2018) Development of a HPLC-MS/MS Method to determine 11 bioactive compounds in Tongmai Yangxin pill and application to a pharmacokinetic study in rats. Evi Based Complement Alternat Med 2018:6460393. https://doi.org/10.1155/2018/6460393
Song XH (2018) Anti-liver cancer activity and mechanism of glycycoumarin. China Agricultural University. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CDFDLAST2019&filename=1018274991.nh
Song XH, Yin ST, Huo YZ, Liang M, Fan LH, Ye M, Hu HB (2015) Glycycoumarin ameliorates alcohol-induced hepatotoxicity via activation of Nrf2 and autophagy. Free Radic Biol Med 89:135–146. https://doi.org/10.1016/j.freeradbiomed.2015.07.006
Song XH, Yin ST, Zhang EX, Fan LH, Ye M, Zhang Y, Hu HB (2016) Glycycoumarin exerts anti-liver cancer activity by directly targeting T-LAK cell-originated protein kinase. Oncotarget 7:65732–65743. https://doi.org/10.18632/oncotarget.11610
Song YQ, Guan XQ, Weng ZM, Liu JL, Chen J, Wang L, Cui LT, Fang SQ, Hou J, Ge GB (2021) Discovery of hCES2A inhibitors from Glycyrrhiza inflata via combination of docking-based virtual screening and fluorescence-based inhibition assays. Food Funct 12:162–176. https://doi.org/10.1039/d0fo02140g
Takeda H, Muto S, Nakagawa K, Ohnishi S, Asaka M (2012) Rikkunshito and ghrelin secretion. Curr Pharm Des 18:4827–4838. https://doi.org/10.2174/138161212803216933
Takeda H, Sadakane C, Hattori T, Katsurada T, Ohkawara T, Nagai K, Asaka M (2008) Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology 134:2004–2013. https://doi.org/10.1053/j.gastro.2008.02.078
Tanaka Y, Kikuzak H, Fukuda S, Nakatani N (2001) Antibacterial compounds of licorice against upper airway respiratory tract pathogens. J Nutr Sci Vitaminol 47:270–273. https://doi.org/10.3177/jnsv.47.270
Tao S, Liang XY, Wang Y, Wang Y (2015) Screening of active compounds with myocardial protective effects from Tongmai Yangxin pill. Zhejiang Da Xue Xue Bao Yi Xue Ban 44:145–153
Uchiumi F, Hatano T, Ito H, Yoshida T, Tanuma S-i (2003) Transcriptional suppression of the HIV promoter by natural compounds. Antiviral Res 58:89–98. https://doi.org/10.1016/s0166-3542(02)00186-9
Van Breemen RB, Huang K, Li G, Huang L, Nikolic D (2014) Safety of botanical dietary supplements used by menopausal women: In vitro investigations of drug-botanical interactions. Planta Med 80:751–751. https://doi.org/10.1055/s-0034-1382298
Wahyuni TS, Widyawaruyanti A, Lusida MI, Fuad A, Soetjipto FH, Kawahara N, Hayashi Y, Aoki C, Hotta H (2014) Inhibition of hepatitis C virus replication by chalepin and pseudane IX isolated from Ruta angustifolia leaves. Fitoterapia 99:276–283. https://doi.org/10.1016/j.fitote.2014.10.011
Wang LL (2016) Research progress on methods of improving bioavailability of insoluble drugs. Continuing Medical Education 30:143–144. https://doi.org/10.3969/j.issn.1004-6763.2016.10.082
Wang LP, Tang XQ (2021) Study on early warning signals of acute lung injury. Life Sci Res 25:1–10. https://doi.org/10.16605/j.cnki.1007-7847.2020.07.0224
Wang Q, Kuang Y, Song W, Qian Y, Qiao X, Guo DA, Ye M (2017) Permeability through the Caco-2 cell monolayer of 42 bioactive compounds in the TCM formula Gegen-Qinlian Decoction by liquid chromatography tandem mass spectrometry analysis. J Pharm Biomed Anal 146:206–213. https://doi.org/10.1016/j.jpba.2017.08.042
Wang Q, Qiao X, Liu CF, Ji S, Feng LM, Qian Y, Guo DA, Ye M (2014) Metabolites identification of glycycoumarin, a major bioactive coumarin from licorice in rats. J Pharm Biomed Anal 98:287–295. https://doi.org/10.1016/j.jpba.2014.06.001
Wang Q, Song W, Qiao X, Ji S, Kuang Y, Zhang ZX, Bo T, Guo DA, Ye M (2016a) Simultaneous quantification of 50 bioactive compounds of the traditional Chinese medicine formula Gegen-Qinlian decoction using ultra-high performance liquid chromatography coupled with tandem mass spectrometry. J Chromatogr A 1454:15–25. https://doi.org/10.1016/j.chroma.2016.05.056
Wang SF, Wang HQ, Liu YN, Wang Y, Fan XH, Cheng YY (2016b) Rapid discovery and identification of anti-inflammatory constituents from traditional Chinese medicine formula by activity index, LC-MS, and NMR. Sci Rep 6:31000. https://doi.org/10.1038/srep31000
Wang SQ (2018) The Management Specification of Health Education in Hospital. Ningbo: Ningbo Publishing House. https://epub.sslibrary.com/epub/reader?ssid=96201836&time=1670049283529&enc=e170a84b703799a4ee9077fe94a04834&classifyId=16041103%2Fbook.duxiu.com%2FbookDetail.jsp%3FdxNumber%3D000017380347%26d%3DA64294F74E3FE2280E0FD68851AAC9F1%26timestr%3D1668592307456%26rtype%3D1
Yakabi K, Harada Y, Takayama K, Ro S, Ochiai M, Iizuka S, Hattori T, Wang L, Taché Y (2014) Peripheral α2-β1 adrenergic interactions mediate the ghrelin response to brain urocortin 1 in rats. Psychoneuroendocrinology 50:300–310. https://doi.org/10.1016/j.psyneuen.2014.09.003
Yan MZ, Wang Z, Xia TJ, Jin SW, Liu YG, Hu HB, Chang Q (2021) Enhancement of TEX264-mediated ER-Phagy contributes to the therapeutic effect of glycycoumarin against APA hepatotoxicity in mice. Biomedicines 9:939. https://doi.org/10.3390/biomedicines9080939
Yan MZ, Ye LH, Yin ST, Lu XT, Liu XY, Lu SY, Cui JL, Fan LH, Kaplowitz N, Hu HB (2018) Glycycoumarin protects mice against acetaminophen-induced liver injury predominantly via activating sustained autophagy. Br J Pharmacol 175:3747–3757. https://doi.org/10.1111/bph.14444
Yang R, Li XT, Li JL, Li SC, Zhang M, Tu T, Liu AH (2019) Progress on pharmacokinetic interaction between licorice and chemical drugs. Prog Vet Med 40:96–99
Yang R, Wang LQ, Yuan BC, Liu Y (2015) The pharmacological activities of licorice. Planta Med 81:1654–1669. https://doi.org/10.1055/s-0035-1557893
Yang XW, Xu B, Ran FX, Wang RQ, Wu J, Cui JR (2007) Inhibitory effects of 11 coumarin compounds against growth of human bladder carcinoma cell line E-J in vitro. Zhong Xi Yi Jie He Xue Bao 5:56–60
Yasmeen B, Anna K, Craig AT (2016) Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med 140:345–350. https://doi.org/10.5858/arpa.2015-0519-ra
Ye RG, Gao J, Wang B, Li EH, Zhang Y, Jiang F, Cao R, Hattori M, Wang SM, Ma CM (2014) Simultaneous quantification and comparison of 8 components in different parts of Glycyrrhiza uralens using ultra-high performance liquid chromatography-triple quadrupole mass spectrometry. Food Sci 35:242–247. https://doi.org/10.7506/spkx1002-6630-201420048
Yin L, Guan ES, Zhang YB, Shu ZH, Wang B, Wu XL, Chen J, Liu JX, Fu XY, Sun WH, Liu MF (2018) Chemical profile and anti-inflammatory activity of total flavonoids from Glycyrrhiza uralensis Fisch. Iran J Pharm Res 17:726–734
Zang YM (2020) Pharmacological activities of coumarin compounds in licorice: a review. Nat Prod Commun 15. https://doi.org/10.1177/1934578X20953954
Zeng L, Li SH, Lou ZC (1988) Morphological and histological studies of Chinese licorice. Yao Xue Xue Bao 23:200–208
Zhang EX, Yin S, Zhao S, Zhao C, Yan M, Fan L, Hu H (2020a) Protective effects of glycycoumarin on liver diseases. Phytother Res 34:1191–1197. https://doi.org/10.1002/ptr.6598
Zhang EX, Song XH, Yin ST, Fan LH, Ye M, Hu HB (2017a) Glycycoumarin prevents hepatic steatosis through activation of adenosine 5,-monophosphate (AMP)-activated protein kinase signaling pathway and up-regulation of BTG1/Tob-1. J Funct Foods 34:277–286. https://doi.org/10.1016/j.jff.2017.04.036
Zhang EX, Yin ST, Lu XT, Ye LH, Fan LH, Hu HB (2018) Glycycoumarin sensitizes liver cancer cells to ABT-737 by targeting de novo lipogenesis and TOPK-survivin axis. Nutrients 10:353. https://doi.org/10.3390/nu10030353
Zhang EX, Yin ST, Song XH, Fan LH, Hu HB (2016) Glycycoumarin inhibits hepatocyte lipoapoptosis through activation of autophagy and inhibition of ER stress/GSK-3-mediated mitochondrial pathway. Sci Rep 6:38138. https://doi.org/10.1038/srep38138
Zhang EX, Yin ST, Zhao C, Fan LH, Hu HB (2020b) Involvement of activation of PLIN5-Sirt1 axis in protective effect of glycycoumarin on hepatic lipotoxicity. Biochem Biophys Res Commun 528:7–13. https://doi.org/10.1016/j.bbrc.2020.05.072
Zhang KJ, Gu QL, Yang K, Ming XJ, Wang JX (2017b) Anticarcinogenic effects of α-mangostin: a review. Planta Med 83:188–202. https://doi.org/10.1055/s-0042-119651
Zhang YF (2019) Research progress on pharmacological activities of Glycyrrhiza uralensis Fisch and its active components. Clin J Chinese Med 11:141–142. https://doi.org/10.3969/j.issn.1674-7860.2019.09.059
Zhu DY, Song GQ, Jiang FX, Chang XR, Guo WB (1984) Studies on chemical constitusents of Glycyrrhiza uralensis Fisch. -The structures of isolicofla vonol and glycycoumarin. Acta Chimica Sinica 42:1080–1084. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=HXXB198410010&DbName=CJFQ1984
Funding
This study was financially supported by the science and technology support program of Guizhou province ([2020]4Y118), the State Key Laboratory for Clinical Pharmacy and Drug Research of Bijie (2019–1), and the Natural Science Basic Research Program of Guizhou Province (ZK[2023]561).
Author information
Authors and Affiliations
Contributions
SW conceived and designed the review. YT collected literature information and wrote the manuscript. SO and LY reviewed the manuscript. All authors have read the final manuscript and approved its submission.
Corresponding author
Additional information
The original online version of this article was revised: Funding information was corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, Y., Ou, S., Ye, L. et al. Pharmacological Activities and Pharmacokinetics of Glycycoumarin. Rev. Bras. Farmacogn. 33, 471–483 (2023). https://doi.org/10.1007/s43450-022-00342-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-022-00342-x