Abstract
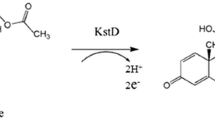
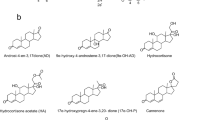
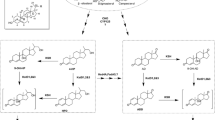
Boldenone is a protein-assimilating androgen steroid that can promote protein synthesis, support nitrogen storage, and enhance renal erythropoietin release. The industrial production of boldenone mainly relies on chemical synthesis, which has various problems, such as a complex conversion process, excessive byproducts, and serious environmental pollution. Therefore, it is of great significance to explore a new biosynthetic route. Recently, the enzymatic synthesis of steroid compounds has been performed more frequently than in the past. In this work, boldenone was produced from androstenedione (AD) in two steps by a dual-enzyme cascade of 17β-hydroxysteroid dehydrogenase (17β-HSD) and 3-sterone-Δ1-dehydrogenase (KstD). The conversion efficiency of three isoenzymes of 17β-HSD from Mycobacterium sp. LY-1 for substrate AD was first analyzed. After that, the 17β-HSD2 with high selectivity and specificity for AD was screened and co-expressed with KstD3 in Escherichia coli BL21 to construct a dual-enzyme catalytic system. The results showed that the synthesis of boldenone from AD could be achieved by constructing the dual-enzyme expression system of 17β-HSD and KstD, as we determined that the concentration of boldenone reached 24.3 mg/L. To further improve the synthesis efficiency of boldenone, the expression conditions of the dual-enzyme system were optimized, and the concentration of boldenone reached 31.9 mg/L. The exploration of this route will provide a foundation for the efficient enzymatic synthesis of boldenone.











Similar content being viewed by others
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Turza A, Miclu MO, Pop A, et al. Crystal and molecular structures of boldenone and four boldenone steroid esters. Zeitschrift für Kristallographie–Crystalline Materials. 2019;234(10):671–83. https://doi.org/10.1515/zkri-2019-0030.
Schüle C, Eser D, Baghai TC, et al. Neuroactive steroids in affective disorders: Target for novel antidepressant or anxiolytic drugs. Neuroscience. 2011;191:55–77. https://doi.org/10.1016/j.neuroscience.2011.03.025.
Xu ZH, Wu Y, Li H, et al. Current state and progress of biotransformation technology of steroidcompounds. Chin J Bioprocess Eng. 2013;11(002):30–6.
Xi ZB, Xue WW, Liu WD, Wang YZ. A high-yield synthesis method of boldenone. Hubei: CN103030677A,2013.
O’Callaghan Y, McCarthy FO, O’Brien NM. Recent advances in phytosterol oxidation products. Biochem Biophys Res Commun. 2014;446(3):786–91. https://doi.org/10.1016/j.bbrc.2014.01.148.
Hang YQ, Wang DQ. Advances in microbial transformation of phytosterol in to steroid medicine intermediates. Microbiol China. 2006;2:142–6.
Eisa M, El-Refai H, Amin M. Single step biotransformation of corn oil phytosterols to boldenone by a newly isolated Pseudomonas aeruginosa. Biotechnol Rep. 2016;11:36–43. https://doi.org/10.1016/j.btre.2016.05.002.
Tang R, Shen Y, Xia M, et al. A highly efficient step-wise biotransformation strategy for direct conversion of phytosterol to boldenone. Biores Technol. 2019;283:242–50. https://doi.org/10.1016/j.biortech.2019.03.058.
Wu YL, Shao ML, Zhou WL, et al. Study on catalytic synthesis of boldenone by recombinant E. coli expressing 17β-hydroxysteroid dehydrogenase. CIESC J. 2020;71(7):3229–37.
Fernández-Cabezón L, Galán B, García JL. Engineering Mycobacterium smegmatis for testosterone production. Microb Biotechnol. 2017;10(1):151–61. https://doi.org/10.1111/1751-7915.12433.
Fernandes P, Cruz A, Angelova B, et al. Microbial conversion of steroid compounds: recent developments. Enzyme Microb Technol. 2003;32(6):688–705. https://doi.org/10.1016/S0141-0229(03)00029-2.
Wojcik P, Glanowski M, Wojtkiewicz AM, et al. Universal capability of 3-ketosteroid Delta(1)-dehydrogenases to catalyze Delta(1)-dehydrogenation of C17-substituted steroids. Microb Cell Fact. 2021;20(1):12. https://doi.org/10.1186/s12934-021-01611-5.
Li Y, Lu F, Sun T, et al. Expression of ksdD gene encoding 3-ketosteroid-Δ 1 -dehydrogenase from Arthrobacter simplex in Bacillus subtilis. Lett Appl Microbiol. 2007;44(5):563–8. https://doi.org/10.1111/j.1472-765X.2007.02134.x.
Zhang WQ, Shao ML, Rao ZM, et al. Bioconversion of 4-androstene-3,17-dione to androst-1,4-diene-3, 17-dione by recombinant Bacillus subtilis expressing ksdd gene encoding 3-ketosteroid-Δ1-dehydrogenase from Mycobacterium neoaurum JC-12. J Steroid Biochem Mol Biol. 2013;135:36–42. https://doi.org/10.1016/j.jsbmb.2012.12.016.
Wang W. Expression and application of 17β- hydroxysteroid dehydrogenase 10(17β-HSD10); Changchun university of science and technology, 2013.
Sultana N. Microbial biotransformation of bioactive and clinically useful steroids and some salient features of steroids and biotransformation. Steroids, 2018, S0039128X18300151. https://doi.org/10.1016/j.steroids.2018.01.007.
Donova MV, Egorova OV, Nikolayeva VM. Steroid 17β-reduction by microorganisms - a review. Process Biochem. 2005;40(7):2253–62. https://doi.org/10.1016/j.procbio.2004.09.025.
BronislavaČrešnar M-M. Aspects of the steroid response in fungi. Chem Biol Interact. 2009;178(1–3):303–9. https://doi.org/10.1016/j.cbi.2008.11.002.
Faramarzi MA, et al. Microbial conversion of androst-1,4-dien-3,17-dione by Mucor racemosus to hydroxysteroid-1,4-dien-3-one derivatives. J Chem Technol Biotechnol. 2009;84(7):1021–5. https://doi.org/10.1002/jctb.2128.
Wang PP, Zheng DN, Peng WL, et al. Characterization of 17-hydroxysteroid dehydrogenase and regulators involved in estrogen degradation in Pseudomonas putida SJTE-1. Appl Microbiol Biotechnol. 2019;103(5):2413–25. https://doi.org/10.1007/s00253-018-9543-y.
Xu LQ, Liu YJ, Yao K, et al. Unraveling and engineering the production of 23,24-bisnorcholenic steroids in sterol metabolism. Sci Rep. 2016;6:21928. https://doi.org/10.1038/srep21928.
Chen MM, Wang FQ, Lin LC, et al. Characterization and application of fusidane antibiotic biosynethsis enzyme 3-ketosteroid- 1 -dehydrogenase in steroid transformation. Appl Microbiol Biotechnol. 2012;96(1):133–42. https://doi.org/10.1007/s00253-011-3855-5.
Kavanagh KL, Jrnvall H, Persson B, et al. The SDR superfamily: functional and structural diversity within a family of metabolic and regulatory enzymes. Cell Mol Life Sci. 2008;65(24):3895–906. https://doi.org/10.1007/s00018-008-8588-y.
Ma Y, Wang XD, Wang MH, et al. Mutation breeding of high 9α- hydroxy- androst- 4- ene- 3,17- dione transforming strains from phytosterols and their conversion process optimization. Chin J Biotechnol. 2017;33(007):1198–206.
Horinouchi M, Hayashi T, Koshino H, et al. Gene Encoding the Hydrolase for the Product of the meta-Cleavage Reaction in Testosterone Degradation by Comamonas testosteroni. Appl Environ Microbiol. 2003;69(4):2139–52. https://doi.org/10.1128/AEM.69.4.2139-2152.2003.
Sambyal K, Singh RV. Production aspects of testosterone by microbial biotransformation and future prospects. Steroids. 2020;159:108651. https://doi.org/10.1016/j.steroids.2020.108651.
Kim K, Kim H, Lee K, et al. Two-promoter vector is highly efficient for overproduction of protein complexes. Protein Sci. 2004;13(6):1698–703. https://doi.org/10.1110/ps.04644504.
Lz A, Hui LA, Yx A, et al. Effects of a nonionic surfactant TX-40 on 9α-hydroxyandrost-4-ene-3, 17-dione biosynthesis and physiological properties of Mycobacterium sp LY-1. Process Biochem. 2019;87:89–94. https://doi.org/10.1016/j.procbio.2019.09.018.
Acknowledgements
Not applicable.
Funding
This project was supported by National Key Research and Development Program of China (No. 2019YFA0905300); the National Natural Science Foundation of China (No. 22078126); Qing Lan Project in Jiangsu Province; Fundamental Research Funds for Central Universities of China (No. JUSRP221025).
Author information
Authors and Affiliations
Contributions
Liang Y carried out the studies, participated in the sequence alignment and drafted the manuscript. Zhang JX, Xu LY participated in the sequence alignment. Liu W participated in the design of the study and performed the statistical analysis. Li H, Shi JS, and Xu ZH conceived of the study, and participated in its design and coordination and helped to draft the manuscript. Chen LY and Wang SL provided some of the experimental material. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
Written informed consent for publication of this paper was obtained from all authors.
Consent for publication
Written informed consent was obtained from authors for publication of this paper and any accompanying images.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, Y., Li, H., Liu, W. et al. Construction and optimization of boldenone synthesis from androstenedione catalyzed by a dual-enzyme system. Syst Microbiol and Biomanuf 4, 783–793 (2024). https://doi.org/10.1007/s43393-023-00187-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-023-00187-y