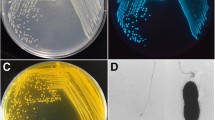
Abstract
Vibrio harveyi is a Gram-negative, rod-shaped, polar flagellate, facultatively anaerobic, halophilic, bioluminescent marine bacteria that belongs to the family of Vibrionaceae, class, Gammaproteobacteria. This pathogenic organism is responsible for various diseases of vertebrates and invertebrates in marine habitats, including shrimp aquaculture. Various symptoms like lesions, gastroenteritis, skin ulcers, eye lesions, and tail rot have been observed due to V. harveyi infection. The pathogenicity mechanism of V. harveyi involves endotoxin lipopolysaccharide, extracellular proteases, and bacteriophage interaction. Hemolysin genes encoded extracellular hemolysin-like phospholipase B toxin could inactivate fish species via the caspase inactivation pathway, ultimately leading to apoptosis. In addition, VBNC (viable but nonculturable) cells are another basis of vibriosis outbreaks in the shrimp aquaculture sector. The extensive amount of antibiotic use promotes the generation of multidrug-resistant strains. Therefore, as an alternative strategy to combat V. harveyi infection, bacteriophages are utilized as a biocontrol agent. However, there is a lack of research on the immobilization and development of encapsulation strategies of V. harveyi-infecting bacteriophages which need to be studied further. In conclusion, the pathogenicity of V. harveyi and its biocontrol by bacteriophages has been documented in this review.

Similar content being viewed by others
Data availability
Not applicable.
References
Atujona D, Cai S, Amenyogbe E. Mini review on Vibrio Infection-a case study on Vibrio harveyi clade. Fish Aquac J. 2018;9(4):1A-A.
Zhang X-H, He X, Austin B. Vibrio harveyi: a serious pathogen of fish and invertebrates in mariculture. Mar Life sci Technol. 2020;2(3):231–45.
Harris L, Owens L. Production of exotoxins by two luminous Vibrio harveyi strains known to be primary pathogens of Penaeus monodon larvae. Dis Aquat Org. 1999;38(1):11–22.
Ruangpan L, Danayadol Y, Direkbusarakom S, Siurairatana S, Flegel T. Lethal toxicity of Vibrio harveyi to cultivated Penaeus monodon induced by a bacteriophage. Dis Aquat Org. 1999;35(3):195–201.
Oakey H, Owens L. A new bacteriophage, VHML, isolated from a toxin-producing strain of Vibrio harveyi in tropical Australia. J Appl Microbiol. 2000;89(4):702–9.
Benala M, Vaiyapuri M, Sivam V, Raveendran K, Mothadaka MP, Badireddy MR. Genome characterization and infectivity potential of vibriophage-ϕLV6 with lytic activity against luminescent Vibrios of Penaeus vannamei shrimp aquaculture. Viruses. 2023;15(4):868.
Austin B, Zhang XH. Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates. Lett Appl Microbiol. 2006;43(2):119–24.
Elias NA, Abu Hassan MS, Yusoff NAH, Tosin OV, Harun NA, Rahmah S, et al. Potential and limitation of biocontrol methods against vibriosis: a review. Aquac Int. 2023. https://doi.org/10.1007/s10499-023-01091-x.
Moriarty D. Control of luminous Vibrio species in penaeid aquaculture ponds. Aquaculture. 1998;164(1–4):351–8.
Cano-Gomez A, Bourne DG, Hall MR, Owens L, Høj L. Molecular identification, typing and tracking of Vibrio harveyi in aquaculture systems: current methods and future prospects. Aquaculture. 2009;287(1–2):1–10.
Frans I, Michiels CW, Bossier P, Willems K, Lievens B, Rediers H. Vibrio anguillarum as a fish pathogen: virulence factors, diagnosis and prevention. J Fish Dis. 2011;34(9):643–61.
Sunaryanto A, Mariam A. Occurrence of a pathogenic bacteria causing luminescence in penaeid larvae in Indonesian hatcheries. Bull Brackishwater Aquac Dev Cent. 1986;8:64–70.
Yanuhar U, Nurcahyo H, Widiyanti L, Junirahma NS, Caesar NR, Sukoso S. In vivo test of Vibrio alginolyticus and Vibrio harveyi infection in the humpback grouper (Cromileptes altivelis) from East Java Indonesia. Vet World. 2022;15(5):1269.
Lavilla-Pitogo CR, Baticados MCL, Cruz-Lacierda ER, Leobert D. Occurrence of luminous bacterial disease of Penaeus monodon larvae in the Philippines. Aquaculture. 1990;91(1–2):1–13.
De Mesa CA, Mendoza RM, Penir SMU, Dela Pena LD, Amar EC, Saloma CP. Genomic analysis of Vibrio harveyi strain PH1009, a potential multi-drug resistant pathogen due to acquisition of toxin genes. Heliyon. 2023.
Karunasagar I, Pai R, Malathi G, Karunasagar I. Mass mortality of Penaeus monodon larvae due to antibiotic-resistant Vibrio harveyi infection. Aquaculture. 1994;128(3–4):203–9.
Jiravanichpaisal P, Miyazaki T, Limsuwan C. Histopathology, biochemistry, and pathogenicity of Vibrio harveyi infecting black tiger prawn Penaeus monodon. J Aquat Anim Health. 1994;6(1):27–35.
Guzman JPMD, Yatip P, Soowannayan C, Maningas MBB. Piper betle L. leaf extracts inhibit quorum sensing of shrimp pathogen Vibrio harveyi and protect Penaeus vannamei postlarvae against bacterial infection. Aquaculture. 2022;547:737452.
Pizzutto M, Hirst RG. Classification of isolates of Vibrio harveyi virulent to Penaeus monodon larvae by protein profile analysis and M13 DNA fingerprinting. Dis Aquat Org. 1995;21(1):61–8.
Samsing F, Zhang W, Zadoks RN, Whittington R, Venturini C, Giles C, et al. Cold temperature stress and damaged skin induced high mortality in barramundi (Lates calcarifer) challenged with Vibrio harveyi. J Fish Dis. 2023. https://doi.org/10.1111/jfd.13784.
Liuxy PC, Lee KK, Chen SN. Pathogenicity of different isolates of Vibrio harveyi in tiger prawn, Penaeus monodon. Lett Appl Microbiol. 1996;22(6):413–6.
Giovanni A, Maekawa S, Wang P-C, Chen S-C. Recombinant Vibrio harveyi flagellin a protein and partial deletions of middle variable region and D0 domain induce immune related genes in Epinephelus coioides and Cyprinus carpio. Dev Comp Immunol. 2023;139: 104588.
Defoirdt T, Sorgeloos P, Bossier P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr Opin Microbiol. 2011;14(3):251–8.
Carlton RM. Phage therapy: past history and future prospects. Arch Immunol Ther Exp. 1999;47:267–74.
Imbeault S, Parent S, Lagacé M, Uhland CF, Blais J-F. Using bacteriophages to prevent furunculosis caused by Aeromonas salmonicida in farmed brook trout. J Aquat Anim Health. 2006;18(3):203–14.
Mathur M, Vidhani S, Mehndiratta P, Bhalla P, Reddy B. Bacteriophage therapy: an alternative to conventional antibiotics. J Assoc Physicians India. 2003;51:593–6.
Łobocka M, Dąbrowska K, Górski A. Engineered bacteriophage therapeutics: rationale, challenges and future. BioDrugs. 2021;35(3):255–80.
Twort FW. An investigation on the nature of ultra-microscopic viruses. Acta Kravsi. 1961.
d’Herelle F. On an invisible microbe antagonistic to dysentery bacilli. CR Acad Sci Paris. 1917;165:373–5.
Surekhamol I, Deepa G, Somnath Pai S, Sreelakshmi B, Varghese S, Bright SI. Isolation and characterization of broad spectrum bacteriophages lytic to Vibrio harveyi from shrimp farms of Kerala. India Lett Appl Microbiol. 2014;58(3):197–204.
Wall SK, Zhang J, Rostagno MH, Ebner PD. Phage therapy to reduce preprocessing Salmonella infections in market-weight swine. Appl Environ Microbiol. 2010;76(1):48–53.
Atterbury RJ, Van Bergen M, Ortiz F, Lovell M, Harris J, De Boer A, et al. Bacteriophage therapy to reduce Salmonella colonization of broiler chickens. Appl Environ Microbiol. 2007;73(14):4543–9.
Huff W, Huff G, Rath N, Balog J, Donoghue A. Alternatives to antibiotics: utilization of bacteriophage to treat colibacillosis and prevent foodborne pathogens. Poult Sci. 2005;84(4):655–9.
Leverentz B, Conway WS, Alavidze Z, Janisiewicz WJ, Fuchs Y, Camp MJ, et al. Examination of bacteriophage as a biocontrol method for Salmonella on fresh-cut fruit: a model study. J Food Prot. 2001;64(8):1116–21.
Bruttin A, Brüssow H. Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob Agents Chemother. 2005;49(7):2874–8.
Wang J, Hu B, Xu M, Yan Q, Liu S, Zhu X, et al. Use of bacteriophage in the treatment of experimental animal bacteremia from imipenem-resistant Pseudomonas aeruginosa. Int J Mol Med. 2006;17(2):309–17.
Kumari S, Harjai K, Chhibber S. Efficacy of bacteriophage treatment in murine burn wound infection induced by Klebsiella pneumoniae. J Microbiol Biotechnol. 2009;19(6):622–8.
Santos T, Gilbert R, Caixeta L, Machado V, Teixeira L, Bicalho R. Susceptibility of Escherichia coli isolated from uteri of postpartum dairy cows to antibiotic and environmental bacteriophages. Part II: in vitro antimicrobial activity evaluation of a bacteriophage cocktail and several antibiotics. J Dairy Sci. 2010;93(1):105–14.
Sundar MM, Nagananda G, Das A, Bhattacharya S, Suryan S. Isolation of host-specific bacteriophages from sewage against human pathogens. Asian J Biotechnol. 2009;1(4):163–70.
Abdulla H, Khafagi I, El-Kareem M, Dewedar A. Bacteriophages in engineered wetland for domestic wastewater treatment. Res J Microbiol. 2007;2(12):889–99.
Pasharawipas T, Thaikua S, Sriurairatana S, Ruangpan L, Direkbusarakum S, Manopvisetcharean J, et al. Partial characterization of a novel bacteriophage of Vibrio harveyi isolated from shrimp culture ponds in Thailand. Virus Res. 2005;114(1–2):63–9.
Shivu MM, Rajeeva BC, Girisha SK, Karunasagar I, Krohne G, Karunasagar I. Molecular characterization of Vibrio harveyi bacteriophages isolated from aquaculture environments along the coast of India. Environ Microbiol. 2007;9(2):322–31.
Kar P, Das TK, Ghosh S, Pradhan S, Chakrabarti S, Mondal KC, et al. Characterization of a Vibrio-infecting bacteriophage, VPMCC5, and proposal of its incorporation as a new genus in the Zobellviridae family. Virus Res. 2022;321: 198904.
Prayitno SB, Latchford J. Experimental infections of crustaceans with luminous bacteria related to Photobacterium and Vibrio. Effect of salinity and pH on infectiosity. Aquaculture. 1995;132(1–2):105–12.
Robertson P, Calderon J, Carrera L, Stark J, Zherdmant M, Austin B. Experimental Vibrio harveyi infections in Penaeus vannamei larvae. Dis Aquat Org. 1998;32(2):151–5.
Nishimori E, Hasegawa O, Numata T, Wakabayashi H. Vibrio carchariae causes mass mortalities in Japanese abalone. Sulculus diversicolor supratexta Fish pathol. 1998;33(5):495–502.
Becker P, Gillan D, Lanterbecq D, Jangoux M, Rasolofonirina R, Rakotovao J, et al. The skin ulceration disease in cultivated juveniles of Holothuria scabra (Holothuroidea, Echinodermata). Aquaculture. 2004;242(1–4):13–30.
Bertone S, Gili C, Moizo A, Calegari L. Vibrio carchariae associated with a chronic skin ulcer on a shark, Carcharhinus plumbeus (Nardo). J Fish Dis. 1996;19(6):429–34.
Hashem M, El-Barbary M. Vibrio harveyi infection in Arabian surgeon fish (Acanthurus sohal) of Red sea at Hurghada, Egypt. Egypt J Aquat Res. 2013;39(3):199–203.
Joseph S, Huxley A, Lipton A. Pathogenicity, antibiogram and biochemical characteristics of luminescent Vibrio harveyi, associated with’black shell disease’of Penaeus monodon. Fish Technol. 2005;42(2):191–6.
Raj ST, Lipton A, Chauhan G. Characterization and infectivity evaluation of Vibrio harveyi causing white patch disease among captive reared seahorses, Hippocampus kuda. 2010.
Zhou J, Fang W, Yang X, Zhou S, Hu L, Li X, et al. A nonluminescent and highly virulent Vibrio harveyi strain is associated with “bacterial white tail disease” of Litopenaeus vannamei shrimp. PLoS ONE. 2012;7(2): e29961.
Muthukrishnan S, Defoirdt T, Ina-Salwany M, Yusoff FM, Shariff M, Ismail SI, et al. Vibrio parahaemolyticus and Vibrio harveyi causing Acute Hepatopancreatic Necrosis Disease (AHPND) in Penaeus vannamei (Boone, 1931) isolated from Malaysian shrimp ponds. Aquaculture. 2019;511: 734227.
Grimes DJ, Stemmler J, Hada H, May E, Maneval D, Hetrick F, et al. Vibrio species associated with mortality of sharks held in captivity. Microb Ecol. 1984;10(3):271–82.
Colwell R, Grimes D. Vibrio diseases of marine fish populations. Helgoländer Meeresuntersuchungen. 1984;37(1):265–87.
Kraxberger-Beatty T, McGarey D, Grier H, Lim D. Vibrio harveyi, an opportunistic pathogen of common snook, Centropomus undecimalis (Bloch), held in captivity. J Fish Dis. 1990;13(6):557–60.
Ishimaru K, Muroga K. Taxonomical re-examination of two pathogenic Vibrio species isolated from milkfish and swimming crab. Fish Pathol. 1997;32(1):59–64.
Hispano C, Nebra Y, Blanch AR. Isolation of Vibrio harveyi from an ocular lesion in the short sunfish (Mola mola). Bull Eur Assoc Fish Pathol. 1997;17:104–7.
Lee K, Liu P-C, Chuang W. Pathogenesis of gastroenteritis caused by Vibrio carchariae in cultured marine fish. Mar Biotechnol. 2002;4(3):267–77.
Soffientino B, Gwaltney T, Nelson DR, Specker JL, Mauel M, Gómez-Chiarri M. Infectious necrotizing enteritis and mortality caused by Vibrio carchariae in summer flounder Paralichthys dentatus during intensive culture. Dis Aquat Org. 1999;38(3):201–10.
Gauger E, Smolowitz R, Uhlinger K, Casey J, Gómez-Chiarri M. Vibrio harveyi and other bacterial pathogens in cultured summer flounder. Paralichthys dentatus Aquac. 2006;260(1–4):10–20.
Yang Q, Xiao G, Chen R, Huang X, Teng S. Immune responses of hemocytes in the blood clam Tegillarca granosa in response to in vivo Vibrio harveyi infection. Fish Shellfish Immunol. 2023;132: 108447.
Saeed M. Association of Vibrio harveyi with mortalities in cultured marine fish in Kuwait. Aquaculture. 1995;136(1–2):21–9.
Zhang XH, Austin B. Pathogenicity of Vibrio harveyi to salmonids. J fish Dis. 2000;23(2):93–102.
Won KM, Park SI. Pathogenicity of Vibrio harveyi to cultured marine fishes in Korea. Aquaculture. 2008;285(1–4):8–13.
Angthong P, Uengwetwanit T, Uawisetwathana U, Koehorst JJ, Arayamethakorn S, Schaap PJ, et al. Investigating host-gut microbial relationship in Penaeus monodon upon exposure to Vibrio harveyi. Aquaculture. 2023;567:739252.
Henke JM, Bassler BL. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J Bacteriol. 2004;186(12):3794–805.
Montánchez I, Kaberdin VR. Vibrio harveyi: A brief survey of general characteristics and recent epidemiological traits associated with climate change. Mar Environ Res. 2020;154: 104850.
Prasad S, Morris PC, Hansen R, Meaden PG, Austin B. A novel bacteriocin-like substance (BLIS) from a pathogenic strain of Vibrio harveyi. Microbiology. 2005;151(9):3051–8.
Eickhoff MJ, Bassler BL. Vibrio fischeri siderophore production drives competitive exclusion during dual-species growth. Mol Microbiol. 2020;114(2):244–61.
Joshi F, Archana G, Desai A. Siderophore cross-utilization amongst rhizospheric bacteria and the role of their differential affinities for Fe 3+ on growth stimulation under iron-limited conditions. Curr Microbiol. 2006;53:141–7.
Owens L, Austin DA, Austin B. Effect of strain origin on siderophore production in Vibrio harveyi isolates. Dis Aquat Org. 1996;27(2):157–60.
McRose DL, Baars O, Seyedsayamdost MR, Morel FM. Quorum sensing and iron regulate a two-for-one siderophore gene cluster in Vibrio harveyi. Proc Natl Acad Sci. 2018;115(29):7581–6.
Zhang X-H. Studies on the pathogenicity mechanisms of the fish pathogen: Heriot-Watt University; 2001.
Nakayama T, Nomura N, Matsumura M. Analysis of the relationship between luminescence and toxicity of Vibrio carchariae pathogenic to shrimp. Fish Sci. 2005;71(6):1236–42.
Yang Q, Han Y, Zhang XH. Detection of quorum sensing signal molecules in the family Vibrionaceae. J Appl Microbiol. 2011;110(6):1438–48.
Defoirdt T, Sorgeloos P. Monitoring of Vibrio harveyi quorum sensing activity in real time during infection of brine shrimp larvae. ISME J. 2012;6(12):2314–9.
Mok KC, Wingreen NS, Bassler BL. Vibrio harveyi quorum sensing: a coincidence detector for two autoinducers controls gene expression. EMBO J. 2003;22(4):870–81.
Manefield M, Harris L, Rice SA, de Nys R, Kjelleberg S. Inhibition of luminescence and virulence in the black tiger prawn (Penaeus monodon) pathogen Vibrio harveyi by intercellular signal antagonists. Appl Environ Microbiol. 2000;66(5):2079–84.
Defoirdt T, Darshanee Ruwandeepika H, Karunasagar I, Boon N, Bossier P. Quorum sensing negatively regulates chitinase in Vibrio harveyi. Environ Microbiol Rep. 2010;2(1):44–9.
Natrah F, Ruwandeepika HD, Pawar S, Karunasagar I, Sorgeloos P, Bossier P, et al. Regulation of virulence factors by quorum sensing in Vibrio harveyi. Vet Microbiol. 2011;154(1–2):124–9.
Lilley BN, Bassler BL. Regulation of quorum sensing in Vibrio harveyi by LuxO and sigma-54. Mol Microbiol. 2000;36(4):940–54.
Donlan RM. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7(2):277.
Guttenplan SB, Kearns DB. Regulation of flagellar motility during biofilm formation. FEMS Microbiol Rev. 2013;37(6):849–71.
Aguilar C, Vlamakis H, Guzman A, Losick R, Kolter R. KinD is a checkpoint protein linking spore formation to extracellular-matrix production in Bacillus subtilis biofilms. MBio. 2010;1(1):e00035-e110.
De Silva L, Heo G-J. Biofilm formation of pathogenic bacteria isolated from aquatic animals. Arch Microbiol. 2023;205(1):36.
Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8(9):881.
Montgomery MT, Kirchman DL. Induction of chitin-binding proteins during the specific attachment of the marine bacterium Vibrio harveyi to chitin. Appl Environ Microbiol. 1994;60(12):4284–8.
Boyd EF. Bacteriophage-encoded bacterial virulence factors and phage–pathogenicity island interactions. Adv Virus Res. 2012;82:91–118.
Arber W. Horizontal gene transfer among bacteria and its role in biological evolution. Life. 2014;4(2):217–24.
Munro J, Oakey J, Bromage E, Owens L. Experimental bacteriophage-mediated virulence in strains of Vibrio harveyi. Dis Aquat Org. 2003;54(3):187–94.
Xu H-S, Roberts N, Singleton F, Attwell R, Grimes DJ, Colwell R. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb Ecol. 1982;8(4):313–23.
Zhang X-H, Ahmad W, Zhu X-Y, Chen J, Austin B. Viable but nonculturable bacteria and their resuscitation: implications for cultivating uncultured marine microorganisms. Mar Life sci Technol. 2021;3:189–203.
Oliver JD. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol Rev. 2010;34(4):415–25.
Orruño M, Parada C, Ogayar E, Kaberdin VR, Arana I. Effects of abiotic and biotic factors on Vibrio harveyi ATCC 14126T survival dynamics in seawater microcosms. Aquat Microb Ecol. 2019;83(2):109–18.
Ramaiah N, Ravel J, Straube W, Hill R, Colwell R. Entry of Vibrio harveyi and Vibrio fischeri into the viable but nonculturable state. J Appl Microbiol. 2002;93(1):108–16.
Li Y, Chen J, Zhao M, Yang Z, Yue L, Zhang X. Promoting resuscitation of viable but nonculturable cells of Vibrio harveyi by a resuscitation-promoting factor-like protein YeaZ. J Appl Microbiol. 2017;122(2):338–46.
Sun F, Chen J, Zhong L, Zhang X-H, Wang R, Guo Q, et al. Characterization and virulence retention of viable but nonculturable Vibrio harveyi. FEMS Microbiol Ecol. 2008;64(1):37–44.
Zhao R, Chen J, Wang Y, Li Y, Kong X, Han Y. Proteolytic activity of Vibrio harveyi YeaZ is related with resuscitation on the viable but non-culturable state. Lett Appl Microbiol. 2020;71(2):126–33.
Deng Y, Xu L, Chen H, Liu S, Guo Z, Cheng C, et al. Prevalence, virulence genes, and antimicrobial resistance of Vibrio species isolated from diseased marine fish in South China. Sci Rep. 2020;10(1):1–8.
Fu S, Ni P, Yang Q, Hu H, Wang Q, Ye S, et al. Delineating the key virulence factors and intraspecies divergence of Vibrio harveyi via whole-genome sequencing. Can J Microbiol. 2021;67(3):231–48.
Waters CM, Bassler BL. The Vibrio harveyi quorum-sensing system uses shared regulatory components to discriminate between multiple autoinducers. Genes Dev. 2006;20(19):2754–67.
Federle MJ, Bassler BL. Interspecies communication in bacteria. J Clin Investig. 2003;112(9):1291–9.
Yang Q, Anh ND, Bossier P, Defoirdt T. Norepinephrine and dopamine increase motility, biofilm formation, and virulence of Vibrio harveyi. Front Microbiol. 2014;5:584.
Travers MA, Basuyaux O, Le Goïc N, Huchette S, Nicolas JL, Koken M, et al. Influence of temperature and spawning effort on Haliotis tuberculata mortalities caused by Vibrio harveyi: an example of emerging vibriosis linked to global warming. Glob Chang Biol. 2009;15(6):1365–76.
Sawabe T, Ogura Y, Matsumura Y, Feng G, Amin AR, Mino S, et al. Updating the Vibrio clades defined by multilocus sequence phylogeny: proposal of eight new clades, and the description of Vibrio tritonius sp. nov. Front Microbiol. 2013;4:414.
Teo JW, Suwanto A, Poh CL. Novel β-lactamase genes from two environmental isolates of Vibrio harveyi. Antimicrob Agents Chemother. 2000;44(5):1309–14.
Shome R, Shome B, Soundararajan R. Studies on luminous Vibrio harveyi isolated from Penaeus monodon larvae reared in hatcheries in Andamans. Indian J Fish. 1999;46(2):141–7.
Yu Y, Tang M, Wang Y, Liao M, Wang C, Rong X, et al. Virulence and antimicrobial resistance characteristics assessment of Vibrio isolated from shrimp (Penaeus vannamei) breeding system in south China. Ecotoxicol Environ Saf. 2023;252: 114615.
Karunasagar I, Otta SK, Karunasagar I. Biofilm formation by Vibrio harveyi on surfaces. Aquaculture. 1996;140(3):241–5.
Jayasree L, Janakiram P, Madhavi R. Characterization of Vibrio spp. associated with diseased shrimp from culture ponds of Andhra Pradesh (India). J World Aquac Soc. 2006;37(4):523–32.
Xu H, Zeng Y-H, Yin W-L, Lu H-B, Gong X-X, Zhang N, et al. Prevalence of bacterial coinfections with Vibrio harveyi in the industrialized flow-through aquaculture systems in Hainan Province: a neglected high-risk lethal causative agent to hybrid grouper. Int J Mol Sci. 2022;23(19):11628.
Gutsell JS. Sulfa drugs and the treatment of furunculosis in trout. Science. 1946;104(2691):85–6.
Lulijwa R, Rupia EJ, Alfaro AC. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: a review of the top 15 major producers. Rev Aquac. 2020;12(2):640–63.
Yano Y, Hamano K, Satomi M, Tsutsui I, Ban M, Aue-Umneoy D. Prevalence and antimicrobial susceptibility of Vibrio species related to food safety isolated from shrimp cultured at inland ponds in Thailand. Food Control. 2014;38:30–6.
Shao ZJ. Aquaculture pharmaceuticals and biologicals: current perspectives and future possibilities. Adv Drug Deliv Rev. 2001;50(3):229–43.
Stalin N, Srinivasan P. Molecular characterization of antibiotic resistant Vibrio harveyi isolated from shrimp aquaculture environment in the south east coast of India. Microb Pathog. 2016;97:110–8.
Gootz TD. The global problem of antibiotic resistance. Crit Rev Immunol. 2010;30(1):79–93.
Hawkey P. The growing burden of antimicrobial resistance. J Antimicrob Chemother. 2008;62(suppl 1):i1–9.
Reygaert WC. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018;4(3):482.
Martínez JL, Baquero F. Interactions among strategies associated with bacterial infection: pathogenicity, epidemicity, and antibiotic resistance. Clin Microbiol Rev. 2002;15(4):647–79.
Avsever M, Türk N, Tunalıgİl S. The increase of antibiotic resistance in aquaculture and its effects on human health. Bornova Veteriner Kontrol Ve Araştirma Enstitüsü Dergisi. 2010;32(46):19–23.
Kang C-H, Kim Y, Oh SJ, Mok J-S, Cho M-H, So J-S. Antibiotic resistance of Vibrio harveyi isolated from seawater in Korea. Mar Pollut Bull. 2014;86(1–2):261–5.
Zhu Z, Dong C, Weng S, He J. The high prevalence of pathogenic Vibrio harveyi with multiple antibiotic resistance in scale drop and muscle necrosis disease of the hybrid grouper, Epinephelus fuscoguttatus (♀)× E. lanceolatus (♂). China J Fish Dis. 2018;41(4):589–601.
Yang A, Li W, Tao Z, Ye H, Xu Z, Li Y, et al. Vibrio harveyi isolated from marine aquaculture species in eastern China and virulence to the large yellow croaker (Larimichthys crocea). J Appl Microbiol. 2021;131(4):1710–21.
Kazi M, Annapure US. Bacteriophage biocontrol of foodborne pathogens. J Food Sci Technol. 2016;53(3):1355–62.
Nakai T, Sugimoto R, Park K-H, Matsuoka S, Mori K-I, Nishioka T, et al. Protective effects of bacteriophage on experimental Lactococcus garvieae infection in yellowtail. Dis Aquat Org. 1999;37(1):33–41.
Liu A, Liu Y, Peng L, Cai X, Shen L, Duan M, et al. Characterization of the narrow-spectrum bacteriophage LSE7621 towards Salmonella enteritidis and its biocontrol potential on lettuce and tofu. LWT. 2020;118: 108791.
Vinod MG, Shivu MM, Umesha K, Rajeeva B, Krohne G, Karunasagar I, et al. Isolation of Vibrio harveyi bacteriophage with a potential for biocontrol of luminous vibriosis in hatchery environments. Aquaculture. 2006;255(1–4):117–24.
Karunasagar I, Shivu M, Girisha S, Krohne G, Karunasagar I. Biocontrol of pathogens in shrimp hatcheries using bacteriophages. Aquaculture. 2007;268(1–4):288–92.
Srinivasan P, Ramasamy P, Brennan G, Hanna R. Inhibitory effects of bacteriophages against shrimp pathogenic Vibrio spp. of the Indian aquaculture environment. Asian J Anim Vet Adv. 2007;2:166–83.
Phumkhachorn P, Rattanachaikunsopon P. Isolation and partial characterization of a bacteriophage infecting the shrimp pathogen Vibrio harveyi. Afr J Microbiol Res. 2010;4(16):1794–800.
Crothers-Stomps C, Høj L, Bourne D, Hall M, Owens L. Isolation of lytic bacteriophage against Vibrio harveyi. J Appl Microbiol. 2010;108(5):1744–50.
Pasharawipas T, Manopvisetcharean J, Flegel T. Phage treatment of Vibrio harveyi: a general concept of protection against bacterial infection. Res J Microbiol. 2011;6(6):560.
Thiyagarajan S, Chrisolite B, Alavandi SV, Poornima M, Kalaimani N, Santiago TC. Characterization of four lytic transducing bacteriophages of luminescent Vibrio harveyi isolated from shrimp (Penaeus monodon) hatcheries. FEMS Microbiol Lett. 2011;325(1):85–91.
Khemayan K, Prachumwat A, Sonthayanon B, Intaraprasong A, Sriurairatana S, Flegel TW. Complete genome sequence of virulence-enhancing Siphophage VHS1 from Vibrio harveyi. Appl Environ Microbiol. 2012;78(8):2790–6.
Patil JR, Desai SN, Roy P, Durgaiah M, Saravanan RS, Vipra A. Simulated hatchery system to assess bacteriophage efficacy against Vibrio harveyi. Dis Aquat Org. 2014;112(2):113–9.
Lal TM, Sano M, Ransangan J. Isolation and characterization of large marine bacteriophage (Myoviridae), VhKM4 infecting Vibrio harveyi. J Aquat Anim Health. 2017;29(1):26–30.
Stalin N, Srinivasan P. Efficacy of potential phage cocktails against Vibrio harveyi and closely related Vibrio species isolated from shrimp aquaculture environment in the south east coast of India. Vet Microbiol. 2017;207:83–96.
Ibrahim WNW, Aznan AS, Saari NA, Leong LK, Musa N, Razzak LA, et al. In-vitro characterization of lytic bacteriophage PhVh6 as potential biocontrol agent against pathogenic Vibrio harveyi. Aquac Aquar Conserv Legis. 2017;10(1):64–76.
Choudhury TG, Maiti B, Venugopal M, Karunasagar I. Influence of some environmental variables and addition of r-lysozyme on efficacy of Vibrio harveyi phage for therapy. J Biosci. 2019;44(1):1–9.
Misol GN Jr, Kokkari C, Katharios P. Biological and genomic characterization of a novel jumbo bacteriophage, vB_VhaM_pir03 with broad host lytic activity against Vibrio harveyi. Pathogens. 2020;9(12):1051.
Wu L, Tian Y, Pang M, Yang Z, Bao H, Zhou Y, et al. A novel vibriophage vB_VhaS_PcB-1G capable of inhibiting virulent Vibrio harveyi pathogen. Aquaculture. 2021;542: 736854.
Cui H, Cong C, Wang L, Li X, Li J, Yang H, et al. Protective effectiveness of feeding phage cocktails in controlling Vibrio harveyi infection of turbot Scophthalmus maximus. Aquaculture. 2021;535: 736390.
Droubogiannis S, Katharios P. Genomic and biological profile of a novel bacteriophage, Vibrio phage virtus, which improves survival of Sparus aurata larvae challenged with Vibrio harveyi. Pathogens. 2022;11(6):630.
Gildea L, Ayariga JA, Robertson BK. Bacteriophages as biocontrol agents in livestock food production. Microorganisms. 2022;10(11):2126.
Zhao Y, An J, Su H, Li B, Liang D, Huang C. Antimicrobial food packaging integrating polysaccharide-based substrates with green antimicrobial agents: a sustainable path. Food Res Int. 2022;155:111096.
Wagh RV, Priyadarshi R, Rhim J-W. Novel bacteriophage-based food packaging: an innovative food safety approach. Coatings. 2023;13(3):609.
Agboluaje M, Sauvageau D. Bacteriophage production in bioreactors. Bacteriophage Ther Methods Mol Biol. 2018;1693:173–93.
Malik DJ. Approaches for manufacture, formulation, targeted delivery and controlled release of phage-based therapeutics. Curr Opin Biotechnol. 2021;68:262–71.
Sauvageau D, Storms Z, Cooper DG. Synchronized populations of Escherichia coli using simplified self-cycling fermentation. J Biotechnol. 2010;149(1–2):67–73.
Sauvageau D, Cooper DG. Two-stage, self-cycling process for the production of bacteriophages. Microb Cell Factories. 2010;9(1):81.
Cinquerrui S, Mancuso F, Vladisavljević GT, Bakker SE, Malik DJ. Nanoencapsulation of bacteriophages in liposomes prepared using microfluidic hydrodynamic flow focusing. Front Microbiol. 2018;9:2172.
Leung SS, Morales S, Britton W, Kutter E, Chan H-K. Microfluidic-assisted bacteriophage encapsulation into liposomes. Int J Pharm. 2018;545(1–2):176–82.
Ma Y, Pacan JC, Wang Q, Sabour PM, Huang X, Xu Y. Enhanced alginate microspheres as means of oral delivery of bacteriophage for reducing Staphylococcus aureus intestinal carriage. Food Hydrocoll. 2012;26(2):434–40.
Ma Y, Pacan JC, Wang Q, Xu Y, Huang X, Korenevsky A, et al. Microencapsulation of bacteriophage Felix O1 into chitosan-alginate microspheres for oral delivery. Appl Environ Microbiol. 2008;74(15):4799–805.
Colom J, Cano-Sarabia M, Otero J, Aríñez-Soriano J, Cortés P, Maspoch D, et al. Microencapsulation with alginate/CaCO3: a strategy for improved phage therapy. Sci Rep. 2017;7(1):41441.
Rosner D, Clark J. Formulations for bacteriophage therapy and the potential uses of immobilization. Pharmaceuticals. 2021;14(4):359.
O’connell L, Marcoux PR, Roupioz Y. Strategies for surface immobilization of whole bacteriophages: a review. ACS Biomater Sci Eng. 2021;7(6):1987–2014.
Chen J, Alcaine SD, Jiang Z, Rotello VM, Nugen SR. Detection of Escherichia coli in drinking water using T7 bacteriophage-conjugated magnetic probe. Anal Chem. 2015;87(17):8977–84.
Jia Y, Qin M, Zhang H, Niu W, Li X, Wang L, et al. Label-free biosensor: a novel phage-modified light addressable potentiometric sensor system for cancer cell monitoring. Biosens Bioelectron. 2007;22(12):3261–6.
Jabrane T, Dubé M, Mangin PJ, (Eds). Bacteriophage immobilization on paper surface: effect of cationic pre-coat layer. Proceedings of Canadian PAPTAC 95th annual meeting. 2009;31–314.
Singh A, Glass N, Tolba M, Brovko L, Griffiths M, Evoy S. Immobilization of bacteriophages on gold surfaces for the specific capture of pathogens. Biosens Bioelectron. 2009;24(12):3645–51.
Tawil N, Sacher E, Mandeville R, Meunier M. Strategies for the immobilization of bacteriophages on gold surfaces monitored by surface plasmon resonance and surface morphology. J Phys Chem C. 2013;117(13):6686–91.
Lone A, Anany H, Hakeem M, Aguis L, Avdjian A-C, Bouget M, et al. Development of prototypes of bioactive packaging materials based on immobilized bacteriophages for control of growth of bacterial pathogens in foods. Int J Food Microbiol. 2016;217:49–58.
Anany H, Chen W, Pelton R, Griffiths M. Biocontrol of Listeria monocytogenes and Escherichia coli O157: H7 in meat by using phages immobilized on modified cellulose membranes. Appl Environ Microbiol. 2011;77(18):6379–87.
Tytgat HL, Schoofs G, Driesen M, Proost P, Van Damme EJ, Vanderleyden J, et al. Endogenous biotin-binding proteins: an overlooked factor causing false positives in streptavidin-based protein detection. Microb Biotechnol. 2015;8(1):164–8.
Ashiani D, Keihan AH, Rashidiani J, Dashtestani F, Eskandari K. Oriented t4 bacteriophage immobilization for recognition of Escherichia coli in capacitance method. Int J Electrochem Sci. 2016;11(12):10087–95.
Tolba M, Minikh O, Brovko L, Evoy S, Griffiths M. Oriented immobilization of bacteriophages for biosensor applications. Appl Environ Microbiol. 2010;76(2):528–35.
Choi I, Chang Y, Kim SY, Han J. Polycaprolactone film functionalized with bacteriophage T4 promotes antibacterial activity of food packaging toward Escherichia coli. Food Chem. 2021;346: 128883.
Mattey M. Treatment of bacterial infections in aquaculture. Patent 10,849,942 B2 U.S. 2020.
Fernández L, Gutiérrez D, Rodríguez A, García P. Application of bacteriophages in the agro-food sector: a long way toward approval. Front Cell Infect Microbiol. 2018;8:296.
Połaska M, Sokołowska B. Bacteriophages—a new hope or a huge problem in the food industry. AIMS Microbiol. 2019;5(4):324.
Zhuhua L, Dezan Y, Yanping Y. Vibrio harveyi giant VP4B and application thereof. Patent CN103555671A Washington, DC: Patent Trademark Office. 2014.
Nagel T, Musila L, Muthoni M, Nikolich M, Nakavuma JL, Clokie MR. Phage banks as potential tools to rapidly and cost-effectively manage antimicrobial resistance in the developing world. Curr Opin Virol. 2022;53: 101208.
Kahn LH, Bergeron G, Bourassa MW, De Vegt B, Gill J, Gomes F, et al. From farm management to bacteriophage therapy: strategies to reduce antibiotic use in animal agriculture. Ann N Y Acad Sci. 2019;1441(1):31–9.
García R, Latz S, Romero J, Higuera G, García K, Bastías R. Bacteriophage production models: an overview. Front Microbiol. 2019;10:1187.
Acknowledgements
The authors are thankful to Dr. Pradip Ghosh, Director, Midnapore City College for providing essential support to carry out this work.
Funding
The authors are grateful to Science and Engineering Research Board (SERB), a statutory body of the Department of Science & Technology, Govt. of India (Sanction Order No. CRG/2019/001750 dated 14th January, 2020) for financial assistance.
Author information
Authors and Affiliations
Contributions
SG: formal analysis, resources, writing—original draft, writing—review and editing. PK: formal analysis, resources, writing—original draft, writing—review and editing. SC: formal analysis, resources, writing—review and editing. SP: formal analysis, resources, writing—review and editing. KCM: formal analysis, resources, writing—review and editing. KG: conceptualization, writing—original draft, writing—review and editing, supervision, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that there is no financial or any other competing interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghosh, S., Kar, P., Chakrabarti, S. et al. Pathogenicity of Vibrio harveyi and its biocontrol using bacteriophages. Syst Microbiol and Biomanuf 3, 552–570 (2023). https://doi.org/10.1007/s43393-023-00178-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-023-00178-z