Abstract
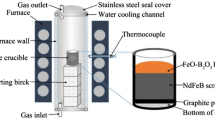
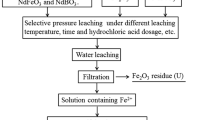
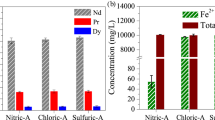
This study proposes a novel waste permanent magnet (WPM) recycling technology by avoiding conventional technology that relies on strong acid and developing a feasible process using relatively inexpensive reagents, thereby reducing overall process cost to acceptable levels. Nd2Fe14B WPMs were transformed into a fluoride/oxide composite material through oxidation/fluorination heat treatment. The phase transition from FeF3-NdF3 to Fe2O3-NdF3 composition was performed via heat treatment. The Fe2O3-NdF3 composition is selectively leached using an oxalic acid. It was confirmed that Fe2O3 was selectively leached, and NdF3 was leached at less than 1 wt.% under various leaching conditions. The neodymium fluoride produced using this technology is expected to be applicable to related fields such as Nd smelting flux or catalysts, and this technology is expected to be applied to various materials containing Fe.







Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper, its supplementary information file.
References
T. Itakura, R. Sasai, H. Itoh, Resource recovery from Nd-Fe-B sintered magnet by hydrothermal treatment. J. Alloys Compd. 408, 1382–1385 (2006). https://doi.org/10.1016/j.jallcom.2005.04.088
C.-H. Lee, Y.-J. Chen, C.-H. Liao, S. R. Popuri, S.-L. Tsai, C.-E. Hung, Selective leaching process for neodymium recovery from scrap Nd-Fe-B magnet. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 44, 5825–5833 (2013). https://doi.org/10.1007/s11661-013-1924-3
T.H. Okabe, O. Takeda, K. Fukuda, Y. Umetsu, Direct extraction and recovery of neodymium metal from magnet scrap. Mater. Trans. 44(4), 798–801 (2003). https://doi.org/10.2320/matertrans.44.798
S. Li, Z. Cui, W. Li, D. Wang, Z. Wang, Technical actuality and prospect of NdFeB waste recycling. Mater. Rep 35, 3001–3009 (2021)
C. Huang, X. Liu, Y. Gao, S. Liu, B. Li, Cathodic processes of neodymium(III) in LiF-NdF3-Nd2O3 melts. Faraday Discuss. 190, 339–349 (2016). https://doi.org/10.1039/c6fd00014B
E. Stefanidaki, C. Hasiotis, C. Kontoyannis, Electrodeposition of neodymium from LiF-NdF–3-Nd2O3 melts. Electrochim. Acta 46(17), 2665–2670 (2001). https://doi.org/10.1016/S0013-4686(01)00489-3
J. Chun, C. Jo, S. Sahgong, M.G. Kim, E. Lim, D.H. Kim, J. Hwang, E. Kang, K.A. Ryu, Y.S. Jung, Y. Kim, J. Lee, Ammonium fluoride mediated synthesis of anhydrous metal fluoride-mesoporous carbon nanocomposites for high-performance lithium ion battery cathodes. ACS Appl. Mater. Interfaces 8(51), 35180–35190 (2016). https://doi.org/10.1021/acsami.6b10641
B. Kozekanan, A. Moradkhani, H. Baharvandi, Thermodynamic and phase analysis of SiC-nano/microB4C-C composites produced by pressureless sintering method. J. Korean Ceram. Soc. 59, 180–192 (2022). https://doi.org/10.1007/s43207-021-00173-x
H. Bae, Y. Shin, L. Mathur, Defect chemistry of p-type perovskite oxide La0.2Sr0.8FeO3-δ: a combined experimental and computational study. J. Korean Ceram. Soc. 59, 876–888 (2022). https://doi.org/10.1007/s43207-022-00237-6
E.P. Lokshin, O. Tareeva, Solubility of YF3, CeF3, PrF3, NdF3, and DyF3 in solutions containing sulfuric and phosphoric acids. Russ. J. Inorg. Chem. 52(12), 1830–1834 (2007). https://doi.org/10.1134/S0036023607120042
K.M. Österdahl, Å.C. Rasmuson, Solubility of β-FeF3·3H2O in mixtures of nitric and hydrofluoric acid. J. Chem. Eng. Data 51(1), 223–229 (2006). https://doi.org/10.1021/je050347n
M. Gergoric, A. Barrier, T. Retegan, Recovery of rare-earth elements from neodymium magnet waste using glycolic, maleic, and ascorbic acids followed by solvent extraction. J. Sustain. Metall. 5, 85–96 (2019). https://doi.org/10.1007/s40831-018-0200-6
G. Reisdörfer, D. Bertuol, E.H. Tanabe, Recovery of neodymium from the magnets of hard disk drives using organic acids. Miner. Eng. 143, 105938 (2019). https://doi.org/10.1016/j.mineng.2019.105938
S.O. Lee, T. Tran, B.H. Jung, S.J. Kim, M.J. Kim, Dissolution of iron oxide using oxalic acid. Hydrometallurgy 87(3–4), 91–99 (2007). https://doi.org/10.1016/j.hydromet.2007.02.005
C. Nwoye, Model for evaluation of the concentration of dissolved phosphorus during leaching of iron oxide ore in oxalic acid solution. JMMCE 8(3), 181–188 (2009). https://doi.org/10.4236/jmmce.2009.83016
R. Salmimies, M. Mannila, J. Kallas, A. Häkkinen, Acidic dissolution of hematite: Kinetic and thermodynamic investigations with oxalic acid. Int. J. Miner. Process. 110, 121–125 (2012). https://doi.org/10.1016/j.minpro.2012.04.001
V. Ambikadevi, M. Lalithambika, Effect of organic acids on ferric iron removal from iron-stained kaolinite. Appl. Clay Sci. 16, 133–145 (2000). https://doi.org/10.1016/S0169-1317(99)00038-1
V. Arslan, A study on the dissolution kinetics of iron oxide leaching from clays by oxalic acid. Physicochem. Probl. Miner. Process, 57 (3), 97–111 (2021). https://doi.org/10.37190/ppmp/135749
F. Chen, F. Liu, L. Wang, J. Wang, Comparison of the preparation process of rare earth oxides from the water leaching solution of waste Nd-Fe-B magnets’ sulfate roasting products. Processes 10(11), 2310 (2022). https://doi.org/10.3390/pr10112310
Q. Liu, T. Tu, H. Guo, H. Cheng, X. Wang, High-efficiency simultaneous extraction of rare earth elements and iron from NdFeB waste by oxalic acid leaching. J. Rare Earths 39(3), 323–330 (2021). https://doi.org/10.1016/j.jre.2020.04.020
Acknowledgements
This work was supported by the Korea Evaluation Institute of Industrial Technology (KEIT), which is funded by the Ministry of Trade, Industry, and Energy, Republic of Korea (Project No. 20015769). This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1A6A1A03045059). We thank the Korea Basic Science Institute for the technical support.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare that are relevant to the content of this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jin, YH., Kim, HS., Yang, JK. et al. Nd composite selective recovery from waste permanent magnet scrap powders by solid-fluorination reaction. J. Korean Ceram. Soc. 61, 97–103 (2024). https://doi.org/10.1007/s43207-023-00350-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-023-00350-0