Abstract
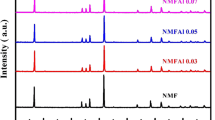
Lithium ions-doped NaV6O15 thin films have been prepared using a simple low temperature liquid phase deposition method and subsequent annealing process. X-ray diffraction (XRD), Fourier transform infrared spectrometer (FTIR), scanning electron microscopy (SEM), and photoelectron spectroscopy (XPS) have been used to study the structural and physicochemical characteristics of the NaV6O15 film. The films were grown on the FTO conductive glass and used directly as an electrode of sodium ion batteries. The prepared lithium ions-doped NaV6O15 thin film electrodes showed an excellent cycling stability and discharge capacity, which may be attributed to the stability of the Li+ embedded into the gap between the V–O layers to maintain the structure and its stable β-phase structure transformed after the first cycle. The cycling stability greatly improved with increasing annealing temperature, while the discharge capacity decreased. The capacities of the film electrodes annealed at 400 °C and 450 °C maintained above 97% after 100 cycles. The lithium-doped NaV6O15 underwent a phase transition during the first charge/discharge cycle. The new transformed phase has perfect crystal structure stability undergoing insertion and deinsertion of Na+. Therefore, the lithium-doped NaV6O15 thin film possesses good cycling stability and is expected to be a promising thin film cathode for sodium-ion batteries.












Similar content being viewed by others
References
S. Liang, J. Zhou, G. Fang, C. Zhang, J. Wu, Y. Tang, A. Pan, Synthesis of mesoporous β-Na0.33V2O5 with enhanced electrochemical performance for lithium ion batteries. Electrochim. Acta. 130, 119–126 (2014)
H. Wu, Y. Zhang, X. Zhang, K. San-Hui, J. Zhu, W. He, Low cost Na2FeSiO4/H–N-doped hard carbon nanosphere hybrid cathodes for high energy and power sodium-ion supercapacitors. J. Alloy. Compd. 842, 155797–155828 (2020)
X. Wang, Q. Liu, H. Wang, D. Jiang, Y. Chang, T. Zhang, B. Zhang, H. Mou, Y. Jiang, PVP-modulated synthesis of NaV6O15 nanorods as cathode materials for high-capacity sodium-ion batteries. J. Mater. Sci. 51, 8986–8994 (2016)
H. Wu, M. Qin, X. Li, Z. Cao, B. Jia, Z. Zhang, D. Zhang, X. Qu, A.A. Volinsky, One step synthesis of vanadium pentoxide sheets as cathodes for lithium ion batteries. Electrochim. Acta. 206, 301–306 (2016)
S. Cao, S. Song, X. Xiang, Q. Hu, C. Zhang, Z. Xia, Y. Xu, W. Zha, J. Li, P.M. Gonzale, Y.H. Han, F. Chen, Modeling, preparation, and elemental doping of Li7La3Zr2O12 garnet-type solid electrolytes: a review. J. Korean Ceram. Soc. 56(2), 111–129 (2019)
R. Arunadevi, B. Kavitha, M. Rajarajan, A. Suganthi, Synthesis of Ce/Mo-V4O9 nanoparticles with superior visible light photocatalytic activity for Rhodamine-B degradation. J. Environ. Chem. Eng. 6, 3349–3357 (2018)
M. Zhao, W. Zhang, X. Song, Lithium-ion storage properties of a micro/nanosheet-like NaV6O15 anode in aqueous solution. Dalton Trans. 46, 3857–3863 (2017)
M. Zhao, W. Zhang, F. Qu, F. Wang, X. Song, Good discharge capacities of NaV6O15 material for an aqueous rechargeable lithium battery. Electrochim. Acta. 138, 187–192 (2014)
F. Hu, W. Jiang, Y. Dong, X. Lai, L. Xiao, X. Wu, Synthesis and electrochemical performance of NaV6O15 microflowers for lithium and sodium ion batteries. RSC Adv. 7, 29481–29488 (2017)
M.D. Soriano, E. Rodríguez-Castellón, E. García-González, J.M. López-Nieto, Catalytic behavior of NaV6O15 bronze for partial oxidation of hydrogen sulfide. Catal. Today. 238, 62–68 (2014)
D. Wu, J. Zeng, H. Hua, J. Wu, Y. Yang, J. Zhao, NaV6O15: a promising cathode material for insertion/extraction of Mg2+ with excellent cycling performance. Nano Res. 13, 335–343 (2020)
Y. Dong, J. Xu, M. Chen, Y. Guo, G. Zhou, N. Li, S. Zhou, C.-P. Wong, Self-assembled NaV6O15 flower-like microstructures for high-capacity and long-life sodium-ion battery cathode. Nano Energy 68, 104357–104374 (2020)
S. Li, Y. Zhang, Y. Tang, X. Tan, S. Liang, J. Zhou, Facile synthesis of LiVO3 and its electrochemical behavior in rechargeable lithium batteries. J. Electroanal. Chem. 853, 113505–113511 (2019)
E.A. Esparcia, M.S. Chae, J.D. Ocon, S.-T. Hong, Ammonium vanadium bronze (NH4V4O10) as a high-capacity cathode material for nonaqueous magnesium–ion batteries. Chem. Mat. 30, 3690–3696 (2018)
Y. Lee, B.U. Ye, D.K. Lee, J.M. Baik, H.K. Yu, M.H. Kim, The migration of alkali metal (Na+, Li+, and K+) ions in single crystalline vanadate nanowires: Rasch–Hinrichsen resistivity. Curr. Appl. Phys. 19, 516–520 (2019)
J. Chen, H.-Y. Xu, J.-H. Ruan, Y.-M. Xin, Y. Li, D.-C. Li, A.-G. Wang, D.-S. Sun, Preparation and electrochemical performance of binder-free sodium vanadium bronze thin film electrodes based on a low temperature liquid phase deposition method. Mater. Chem. Phys. 249, 122935–122945 (2020)
S.H. Gong, J. Lee, H.S. Kim, Development of electrode architecture using Sb–rGO composite and CMC binder for high-performance sodium-ion battery anodes. J. Korean Ceram. Soc. 57(1), 91–97 (2020)
N.U.R. Lashari, M. Zhao, Q. Zheng, H. Gong, X. Song, Good lithium-ion insertion/extraction characteristics of a novel double metal doped hexa-vanadate compounds used in an inorganic aqueous solution. Energy Fuels. 32, 10016–10023 (2018)
I. Seo, G.C. Hwang, J.-K. Kim, Y. Kim, Electrochemical characterization of micro-rod β-Na0.33V2O5 for high performance lithium ion batteries. Electrochim. Acta. 193, 160–165 (2016)
M. Najdoski, V. Koleva, S. Demiri, Chemical bath deposition and characterization of electrochromic thin films of sodium vanadium bronzes. Mater. Res. Bull. 47, 737–743 (2012)
M.-L. Qin, W.-M. Liu, Y.-J. Xiang, W.-G. Wang, B. Shen, Synthesis and electrochemical performance of V2O5/NaV6O15 nanocomposites as cathode materials for sodium-ion batteries. Trans. Nonferr. Met. Soc. China. 30, 2200–2206 (2020)
S. Osman, S. Zuo, X. Xu, J. Shen, Z. Liu, F. Li, P. Li, X. Wang, J. Liu, Freestanding sodium vanadate/carbon nanotube composite cathodes with excellent structural stability and high rate capability for sodium-ion batteries. ACS Appl. Mater. Interfaces. 13, 816–826 (2021)
D. McNulty, D. Noel-Buckley, C. O’Dwyer, NaV2O5 from sodium ion-exchanged vanadium oxide nanotubes and its efficient reversible lithiation as a Li-ion anode material. ACS Appl. Energy Mater. 2, 822–832 (2019)
J. Bard, Electrochemical Methods Fundamentals and Applications, 2nd edn. (Wiley, New York, 2001), p. 159
Y. Lu, J. Wu, J. Liu, M. Lei, S. Tang, P. Lu, L. Yang, H. Yang, Q. Yang, Facile synthesis of Na0.33V2O5 nanosheet-graphene hybrids as ultrahigh performance cathode materials for lithium ion batteries. ACS Appl. Mater. Interfaces. 7, 17433–17441 (2015)
X. Song, J. Li, Z. Li, Q. Xiao, G. Lei, Z. Hu, Y. Ding, H.M. Kheimeh Sari, X. Li, Superior sodium storage of carbon-coated NaV6O15 nanotube cathode: pseudocapacitance versus intercalation. ACS Appl. Mater. Interfaces. 11, 10631–10641 (2019)
Y. Zhu, Y. Xu, Y. Liu, C. Luo, C. Wang, Comparison of electrochemical performances of olivine NaFePO4 in sodium-ion batteries and olivine LiFePO4 in lithium-ion batteries. Nanoscale 5, 780–788 (2013)
L. Deng, X. Niu, G. Ma, Z. Yang, L. Zeng, Y. Zhu, L. Guo, Layered potassium vanadate K0.5V2O5 as a cathode material for nonaqueous potassium ion batteries. Adv. Funct. Mater. 28, 1800670–1800678 (2018)
M. Najdoski, V. Koleva, S. Stojkovikj, T. Todorovski, Electrochromic thin films of sodium intercalated vanadium(V) oxide xerogels: chemical bath deposition and characterization. Surf. Coat. Technol. 277, 308–317 (2015)
C.K. Christensen, D.R. Sørensen, J. Hvam, D.B. Ravnsbæk, Structural evolution of disordered LixV2O5 bronzes in V2O5 cathodes for li-ion batteries. Chem. Mat. 31, 512–520 (2018)
Q. Tan, Q. Zhu, A. Pan, Y. Wang, Y. Tang, X. Tan, S. Liang, G. Cao, Template-free synthesis of β-Na0.33V2O5 microspheres as cathode materials for lithium-ion batteries. CrystEngComm 17, 4774–4780 (2015)
R. Li, C. Guan, X. Bian, X. Yu, F. Hu, NaV6O15 microflowers as a stable cathode material for high-performance aqueous zinc-ion batteries. RSC Adv. 10, 6807–6813 (2020)
H. Liu, Y. Wang, L. Li, K. Wang, E. Hosono, H. Zhou, Facile synthesis of NaV6O15 nanorods and its electrochemical behavior as cathode material in rechargeable lithium batteries. J. Mater. Chem. 19, 7885–7892 (2009)
V. Soundharrajan, B. Sambandam, S. Kim, M.H. Alfaruqi, D.Y. Putro, J. Jo, S. Kim, V. Mathew, Y.K. Sun, J. Kim, Na2V6O16.3H2O barnesite nanorod: an open door to display a stable and high energy for aqueous rechargeable Zn-ion batteries as cathodes. Nano Lett. 18, 2402–2410 (2018)
H.T.T. Nguyen, D. Jung, C.-Y. Park, D.J. Kang, Synthesis of single-crystalline sodium vanadate nanowires based on chemical solution deposition method. Mater. Chem. Phys. 165, 19–24 (2015)
Y. Yang, D. Kim, P. Schmuki, Electrochromic properties of anodically grown mixed V2O5–TiO2 nanotubes. Electrochem. Commun. 13, 1021–1025 (2011)
E.A. Skryleva, I.V. Kubasov, P.V. Kiryukhantsev-Korneev, B.R. Senatulin, R.N. Zhukov, K.V. Zakutailov, M.D. Malinkovich, Y.N. Parkhomenko, XPS study of Li/Nb ratio in LiNbO3 crystals. Effect of polarity and mechanical processing on LiNbO3 surface chemical composition. Appl. Surf. Sci. 389, 387–394 (2016)
Acknowledgements
Financial supports from Excellent Young Talents Fund Program of Higher Education Institutions of Anhui Province (gxyqZD2016150), National Science and Technology Major Project (2019YFE03070001), and the Key Research and Development Projects of Anhui Province (202004b11020033) are acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, H.Y., Ruan, J.H., Liu, F.L. et al. Preparation of lithium-doped NaV6O15 thin film cathodes with high cycling performance in SIBs. J. Korean Ceram. Soc. 59, 289–301 (2022). https://doi.org/10.1007/s43207-021-00127-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-021-00127-3