Abstract
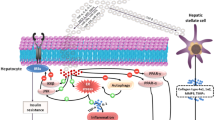
The crosstalk between obesity and insulin resistance (IR) in polycystic ovary syndrome (PCOS) may be related to miRNA regulation secreted by exosomes. However, the underlying mechanism remains to be explored. A model of PCOS with IR was constructed in mice with dehydroepiandrosterone (DHEA) and a high-fat diet (HFD). Serum exosomes were extracted and characterized using transmission electron microscopy (TEM) and western blot analysis (for CD9, CD63, and CD81). The expression of miR-20b-5p and miR-106a-5p in serum exosomes was detected by qRT-PCR. The effects of serum exosomal miR-20b-5p and miR-106a-5p on lipid metabolism and ovary histological structure in PCOS model with IR were also explored. Serum exosomal miR-20b-5p and miR-106a-5p overexpression could inhibit adipocyte differentiation in 3T3-L1 cells with IR and PCOS mice model. Furthermore, the predicted targets of miR-20b-5p and miR-106a-5p were also analyzed with bioinformatics. In DHEA + HFD serum-derived exosomes, the miR-20b-5p and miR-106a-5p levels were markedly decreased. Overexpression of miR-20b-5p and miR-106a-5p alleviated adipocyte differentiation–related genes and triglyceride content in 3T3-L1 cells and liver steatosis in mice. Bioinformatics analysis of miR-20b-5p and miR-106a-5p predicted targets indicated that miR-20b-5p and miR-106a-5p were highly related to lipid metabolism. Serum-derived exosome miR-20b-5p and miR-106a-5p inhibited adipocyte differentiation during the process of PCOS with IR, which might be a novel therapeutic target.






Similar content being viewed by others
Data Availability
The data supporting the findings of this study are available from the corresponding author upon request.
Code Availability
Not applicable.
References
Hoeger KM, Dokras A, Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. J Clin Endocrinol Metab. 2021;106:e1071–83.
Group REASPCW. Revised. consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2003;2004(19):41–7.
Balen AH, Morley LC, Misso M, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22:687–708. https://doi.org/10.1093/humupd/dmw025.
Azziz R. Polycystic Ovary Syndrome. Obstet Gynecol. 2018;132:321–36. https://doi.org/10.1097/AOG.0000000000002698.
De Groot PC, Dekkers OM, Romijn JA, et al. PCOS, coronary heart disease, stroke and the influence of obesity: a systematic review and meta-analysis. Hum Reprod Update. 2011;17:495–500.
Chen L, Xu WM, Zhang D. Association of abdominal obesity, insulin resistance, and oxidative stress in adipose tissue in women with polycystic ovary syndrome. Fertil Steril. 2014;102(1167–1174):e1164.
Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14. https://doi.org/10.1016/j.addr.2015.05.001.
Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126:1208–15. https://doi.org/10.1172/JCI81135.
Pullan JE, Confeld MI, Osborn JK, et al. Exosomes as drug carriers for cancer therapy. Mol Pharm. 2019;16:1789–98. https://doi.org/10.1021/acs.molpharmaceut.9b00104.
Yuan D, Luo J, Sun Y, et al. PCOS follicular fluid derived exosomal miR-424–5p induces granulosa cells senescence by targeting CDCA4 expression. Cell Signal. 2021;85:110030. https://doi.org/10.1016/j.cellsig.2021.110030.
Che X, Jian F, Chen C, et al. PCOS serum-derived exosomal miR-27a-5p stimulates endometrial cancer cells migration and invasion. J Mol Endocrinol. 2020;64:1–12.
Jiang X, Li J, Zhang B, et al. Differential expression profile of plasma exosomal microRNAs in women with polycystic ovary syndrome. Fertil Steril. 2021;115:782–92. https://doi.org/10.1016/j.fertnstert.2020.08.019.
Moghetti P, Tosi F. Insulin resistance and PCOS: chicken or egg? J Endocrinol Invest. 2021;44:233–44.
Li Y, Zhao W, Wang H, et al. Silencing of LncRNA steroid receptor RNA activator attenuates polycystic ovary syndrome in mice. Biochimie. 2019;157:48–56. https://doi.org/10.1016/j.biochi.2018.10.021.
Sun LF, Yang YL, Wang MY, et al. Inhibition of Col6a5 improve lipid metabolism disorder in dihydrotestosterone-induced hyperandrogenic mice. Front Cell Dev Biol. 2021;9:669189. https://doi.org/10.3389/fcell.2021.669189.
Zhang C, Yu C, Lin Z, et al. MiRNAs expression profiling of rat ovaries displaying PCOS with insulin resistance. Arch Gynecol Obstet. 2020;302:1205–13. https://doi.org/10.1007/s00404-020-05730-z.
Qin L, Chen J, Tang L, et al. Significant role of Dicer and miR-223 in adipose tissue of polycystic ovary syndrome patients. Biomed Res Int. 2019;2019:9193236. https://doi.org/10.1155/2019/9193236.
He X, Zhang J. Why do hubs tend to be essential in protein networks? PLoS genetics. 2006;2:e88.
Szklarczyk D, Franceschini A, Wyder S, et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–52.
Wang L, Fan H, Zou Y, et al. Aberrant Expression of long non-coding RNAs in exosomes in follicle fluid from PCOS patients. Front Genet. 2020;11:608178. https://doi.org/10.3389/fgene.2020.608178.
Carmina E, Lobo RA. Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab. 1999;84:1897–9.
Diamanti-Kandarakis E, Papavassiliou AG, Kandarakis SA, et al. Pathophysiology and types of dyslipidemia in PCOS. Trends Endocrinol Metab. 2007;18:280–5.
Silfen ME, Denburg MR, Manibo AM, et al. Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab. 2003;88:4682–8.
Wang M, Zhao D, Xu L, et al. Role of PCSK9 in lipid metabolic disorders and ovarian dysfunction in polycystic ovary syndrome. Metabolism. 2019;94:47–58. https://doi.org/10.1016/j.metabol.2019.02.002.
Castano C, Kalko S, Novials A, et al. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci U S A. 2018;115:12158–63. https://doi.org/10.1073/pnas.1808855115.
Wild RA. Dyslipidemia in PCOS. Steroids. 2012;77:295–9.
Purcell SH, Moley KH. The impact of obesity on egg quality. J Assist Reprod Genet. 2011;28:517–24.
Zhang Y, Li C, Zhang W, et al. Decreased insulin resistance by myo-inositol is associated with suppressed interleukin 6/phospho-STAT3 signaling in a rat polycystic ovary syndrome model. J Med Food. 2020;23:375–87. https://doi.org/10.1089/jmf.2019.4580.
Wekker V, van Dammen L, Koning A, et al. Long-term cardiometabolic disease risk in women with PCOS: a systematic review and meta-analysis. Hum Reprod Update. 2020;26:942–60. https://doi.org/10.1093/humupd/dmaa029.
Patel SS, Truong U, King M, et al. Obese adolescents with polycystic ovarian syndrome have elevated cardiovascular disease risk markers. Vasc Med. 2017;22:85–95. https://doi.org/10.1177/1358863X16682107.
Krishnan A, Muthusami S. Hormonal alterations in PCOS and its influence on bone metabolism. J Endocrinol. 2017;232:R99–113. https://doi.org/10.1530/JOE-16-0405.
Ajmal N, Khan SZ, Shaikh R. Polycystic ovary syndrome (PCOS) and genetic predisposition: a review article. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100060. https://doi.org/10.1016/j.eurox.2019.100060.
Witchel SF, Oberfield SE, Pena AS. Polycystic ovary syndrome: pathophysiology, presentation, and treatment with emphasis on adolescent girls. J Endocr Soc. 2019;3:1545–73. https://doi.org/10.1210/js.2019-00078.
Paz MM, Garcia NE, Semhan RV, et al. Study of lipid reserves in Liolaemus koslowskyi (Squamata: Liolaemidae): reproductive and ecological implications. J Comp Physiol B. 2019;189:595–609. https://doi.org/10.1007/s00360-019-01226-8.
Ferrer MJ, Silva AF, Abruzzese GA, et al. Lipid metabolism and relevant disorders to female reproductive health. Curr Med Chem. 2021;28:5625–47. https://doi.org/10.2174/0929867328666210106142912.
Athyros VG, Doumas M, Imprialos KP, et al. Diabetes and lipid metabolism. Hormones (Athens). 2018;17:61–7. https://doi.org/10.1007/s42000-018-0014-8.
Li Y, Ma J, Yao K, et al. Circadian rhythms and obesity: timekeeping governs lipid metabolism. J Pineal Res. 2020;69:e12682. https://doi.org/10.1111/jpi.12682.
Hjerpsted JB, Flint A, Brooks A, et al. Semaglutide improves postprandial glucose and lipid metabolism, and delays first-hour gastric emptying in subjects with obesity. Diabetes Obes Metab. 2018;20:610–9. https://doi.org/10.1111/dom.13120.
Esfandyari S, Elkafas H, Chugh RM, et al. Exosomes as biomarkers for female reproductive diseases diagnosis and therapy. Int J Mol Sci 2021; 22 https://doi.org/10.3390/ijms22042165.
Sun X, Ma X, Yang X, et al. Exosomes and female infertility. Curr Drug Metab. 2019;20:773–80. https://doi.org/10.2174/1389200220666191015155910.
Lian Y-k and Zhou W-d. Advances in the research of exosome and exosomal non-coding RNA in the pathogenesis, diagnosis and treatment of polycystic ovary syndrome. Acta Pharmaceutica Sinica 2020: 2256–2263.
Wang L, Fan H, Zou Y, et al. Aberrant expression of long noncoding RNAs in exosomes in follicle fluid from PCOS patients. Front Genet. 2020;11:1822.
Zhao Y, Tao M, Wei M, et al. Mesenchymal stem cells derived exosomal miR-323-3p promotes proliferation and inhibits apoptosis of cumulus cells in polycystic ovary syndrome (PCOS). Artif Cells Nanomedicine Biotechnol. 2019;47:3804–13.
Wu HL, Heneidi S, Chuang TY, et al. The expression of the miR-25/93/106b family of micro-RNAs in the adipose tissue of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99:E2754-2761. https://doi.org/10.1210/jc.2013-4435.
Funding
This work was supported by the Youth Project of National Natural Science Foundation of China (grant number 81904237).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
The protocol was approved by the Animal Ethics Committee, Affiliated Hospital of Nanjing University of Chinese Medicine.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hong, Y., Wu, J., Yu, S. et al. Serum-Derived Exosomal microRNAs in Lipid Metabolism in Polycystic Ovary Syndrome. Reprod. Sci. 29, 2625–2635 (2022). https://doi.org/10.1007/s43032-022-00930-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-00930-1