Abstract
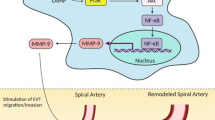
The steroid hormones act by binding to their receptors and subsequently interacting with coactivators. Several classes of coactivators have been identified and shown to be essential in estradiol (E2) responsiveness. The major coregulators are the p160 steroid receptor coactivator (SRC) family. Although the function of SRCs in other organs has been well studied, it has not been thoroughly studied in the placenta. In addition, the correlation between preeclampsia (PE) and SRCs has not been examined previously. Therefore, we compared the expression patterns of SRCs in normal and PE placentas. In human PE placental tissues, SRC-1 mRNA, and protein levels were downregulated in the PE group. In addition, to assess the expression of SRCs in a PE environment, we used Reduced Uterine Perfusion Pressure (RUPP) model and placental cells were cultured in hypoxia condition. SRC-1 proteins were reduced in the placenta of PE-like rat RUPP model. Furthermore, SRCs proteins were significantly downregulated in hypoxia-grown placental cells. To examine the interaction between estrogen receptors (ERs) and SRC-1 protein, we performed co-immunoprecipitation. The interaction of SRC-1 with ERα was significantly stronger than that with ERβ. In PE placenta, the interaction of both ERα and ERβ with SRC-1 was stronger than that in normal placenta. In summary, our results demonstrate that expression levels of SRC-1, not SRC-2 and SRC-3, were decreased in hypoxia-induced PE placenta, which may further reduce the signaling of sex steroid hormones such as E2. The dysregulated signaling of E2 by SRC-1 expression could be associated with the PE placental symptoms of patients.





Similar content being viewed by others
References
Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thromb Res. 2004;114(5–6):397–407.
Ueki N, Takeda S, Koya D, Kanasaki K. The relevance of the renin-angiotensin system in the development of drugs to combat preeclampsia. Int J Endocrinol 2015;2015:572713.
Kumar P, Magon N. Hormones in pregnancy. Nigerian medical journal : journal of the Nigeria Medical Association. 2012;53(4):179–83.
McKenna NJ, O'Malley BW. Minireview: nuclear receptor coactivators--an update. Endocrinology. 2002;143(7):2461–5.
Leo C, Chen JD. The SRC family of nuclear receptor coactivators. Gene. 2000;245(1):1–11.
Stashi E, York B, O’Malley BW. Steroid receptor coactivators: servants and masters for control of systems metabolism. Trends in Endocrinology & Metabolism. 2014;25(7):337–47.
Dasgupta S, Lonard DM, O'Malley BW. Nuclear receptor coactivators: master regulators of human health and disease. Annu Rev Med. 2014;65:279–92.
Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai M-J, O'malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279(5358):1922–5.
Han SJ, DeMayo FJ, Xu J, Tsai SY, Tsai M-J, O’malley BW. Steroid receptor coactivator (SRC)-1 and SRC-3 differentially modulate tissue-specific activation functions of the progesterone receptor. Mol Endocrinol. 2006;20(1):45–55.
Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O'Malley BW. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci. 2000;97(12):6379–84.
Gehin M, Mark M, Dennefeld C, Dierich A, Gronemeyer H, Chambon P. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol Cell Biol. 2002;22(16):5923–37.
Moser M. Working group report on high blood pressure in pregnancy. The Journal of Clinical Hypertension. 2001;3(2):75–88.
Duley L. Pre-eclampsia and the hypertensive disorders of pregnancy. Br Med Bull. 2003;67(1):161–76.
Park Y, Cho GJ, Kim LY, Lee TS, Oh MJ, Kim YH. Preeclampsia increases the incidence of postpartum cerebrovascular disease in Korean population. J Korean Med Sci. 2018;33(6):e35.
Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11(6):342–52.
Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. Am J Obstet Gynecol. 1996;175(5):1365–70.
Fügedi G, Molnár M, Rigó J, Schönléber J, Kovalszky I, Molvarec A. Increased placental expression of cannabinoid receptor 1 in preeclampsia: an observational study. BMC pregnancy and childbirth. 2014;14(1):395–403.
Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–4.
Cantonwine DE, McElrath TF, Trabert B, et al. Estrogen metabolism pathways in preeclampsia and normal pregnancy. Steroids. 2019;144:8–14.
Wan J, Hu Z, Zeng K, et al. The reduction in circulating levels of estrogen and progesterone in women with preeclampsia. Pregnancy hypertension. 2018;11:18–25.
Shin YY, Jeong JS, Park MN, et al. Regulation of steroid hormones in the placenta and serum of women with preeclampsia. Mol Med Rep. 2018;17(2):2681–8.
Fushima T, Sekimoto A, Minato T, et al. Reduced uterine perfusion pressure (RUPP) model of preeclampsia in mice. PLoS One. 2016;11(5):e0155426.
Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Phys Heart Circ Phys. 2012;303(1):H1–8.
An B-S, Selva DM, Hammond GL, Rivero-Muller A, Rahman N, Leung PC. Steroid receptor coactivator-3 is required for progesterone receptor trans-activation of target genes in response to gonadotropin-releasing hormone treatment of pituitary cells. J Biol Chem. 2006;281(30):20817–24.
Ahmed A. New insights into the etiology of preeclampsia: identification of key elusive factors for the vascular complications. Thromb Res. 2011;127:S72–5.
Ahmad S, Hewett PW, Al-Ani B, et al. Autocrine activity of soluble Flt-1 controls endothelial cell function and angiogenesis. Vascular cell. 2011;3(1):1.
Redman C, Sargent I. Pre-eclampsia, the placenta and the maternal systemic inflammatory response—a review. Placenta. 2003;24:S21–7.
Zeisler H, Jirecek S, Hohlagschwandtner M, Knofler M, Tempfer C, Livingston JC. Concentrations of estrogens in patients with preeclampsia. Wien Klin Wochenschr. 2002;114(12):458–61.
Park MN, Park KH, Lee JE, et al. The expression and activation of sex steroid receptors in the preeclamptic placenta. Int J Mol Med. 2018;41(5):2943–51.
Zhou H-J, Yan J, Luo W, et al. SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res. 2005;65(17):7976–83.
Cai D, Shames DS, Raso MG, et al. Steroid receptor coactivator-3 expression in lung cancer and its role in the regulation of cancer cell survival and proliferation. Cancer Res. 2010;70(16):6477–85.
Qin L, Liu Z, Chen H, Xu J. The steroid receptor coactivator-1 regulates twist expression and promotes breast cancer metastasis. Cancer Res. 2009;69(9):3819–27.
Kim SC, Park M-N, Lee YJ, Joo JK, An B-S. Interaction of steroid receptor coactivators and estrogen receptors in the human placenta. J Mol Endocrinol. 2016;56(3):239–47.
Kanasaki K, Palmsten K, Sugimoto H, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453(7198):1117.
Tian M, Zhang Y, Liu Z, Sun G, Mor G, Liao A. The PD-1/PD-L1 inhibitory pathway is altered in pre-eclampsia and regulates T cell responses in pre-eclamptic rats. Sci Rep. 2016;6:27683.
Suzuki H, Ohkuchi A, Shirasuna K, et al. Animal models of preeclampsia: insight into possible biomarker candidates for predicting preeclampsia. Med J Obstet Gynecol. 2014;2(2):1031.
Burleigh D, Kendziorski C, Choi Y, et al. Microarray analysis of BeWo and JEG3 trophoblast cell lines: identification of differentially expressed transcripts. Placenta. 2007;28(5–6):383–9.
Rothbauer M, Patel N, Gondola H, Siwetz M, Huppertz B, Ertl P. A comparative study of five physiological key parameters between four different human trophoblast-derived cell lines. Sci Rep. 2017;7(1):5892.
Schmidt A, Morales-Prieto DM, Pastuschek J, Froehlich K, Markert UR. Only humans have human placentas: molecular differences between mice and humans. J Reprod Immunol. 2015;108:65–71.
Kim J, Lee K-S, Kim J-H, et al. Aspirin prevents TNF-α-induced endothelial cell dysfunction by regulating the NF-κB-dependent miR-155/eNOS pathway: role of a miR-155/eNOS axis in preeclampsia. Free Radic Biol Med. 2017;104:185–98.
Harmon A, Cornelius D, Amaral L, et al. IL-10 supplementation increases Tregs and decreases hypertension in the RUPP rat model of preeclampsia. Hypertension in pregnancy. 2015;34(3):291–306.
Li P, Gan Y, Xu Y, et al. 17beta-estradiol attenuates TNF-α-induced premature senescence of nucleus pulposus cells through regulating the ROS/NF-κB pathway. Int J Biol Sci. 2017;13(2):145.
Acknowledgments
This study was supported by Research institute for Convergence of biomedical science and technology (30-2016-014), Pusan National University Yangsan Hospital. The biospecimens and data used for this study were provided by the Biobank of Pusan National University Hospital, a member of the Korea Biobank Network.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Authors do not have conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Jeong, J.S., Lee, D.H., Lee, JE. et al. The Expression and Contribution of SRCs with Preeclampsia Placenta. Reprod. Sci. 27, 1513–1521 (2020). https://doi.org/10.1007/s43032-020-00142-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00142-5