Abstract
Wheat production requires at least ~ 2.4% increase per year rate by 2050 globally to meet food demands. However, heat stress results in serious yield loss of wheat worldwide. Correspondingly, wheat has evolved genetic basis and molecular mechanisms to protect themselves from heat-induced damage. Thus, it is very urgent to understand the underlying genetic basis and molecular mechanisms responsive to elevated temperatures to provide important strategies for heat-tolerant varieties breeding. In this review, we focused on the impact of heat stress on morphology variation at adult stage in wheat breeding programs. We also summarize the recent studies of genetic and molecular factors regulating heat tolerance, including identification of heat stress tolerance related QTLs/genes, and the regulation pathway in response to heat stress. In addition, we discuss the potential ways to improve heat tolerance by developing new technologies such as genome editing. This review of wheat responses to heat stress may shed light on the understanding heat-responsive mechanisms, although the regulatory network of heat tolerance is still ambiguous in wheat.
Similar content being viewed by others
Introduction
Wheat (Triticum aestivum L.) is the most widely grown staple crop in the world, cultivated from 67° N in Scandinavia and Russia to 45° S in Argentina. It serves as a rich source of proteins, minerals and other essential nutrients for approximately 30% of the human population (IWGSC 2014). Due to the increasing population, wheat production requires ~ 2.4% increase per year to meet global food demands by 2050 (Ray et al. 2013). As a chimonophilous plant, wheat is sensitive to heat stress and prefers an optimal daytime growing temperature of 20–24 °C during reproductive development (Farooq et al. 2011). Model predictions indicate that global wheat production will fall by 6% per 1 °C increase above optimum temperature (Asseng et al. 2015). Since the Industrial Revolution, the average global surface temperature has warmed by 0.85 °C (IWGSC 2014), and this trend will continue and is expected to rise more than 1.5 °C by the end of twenty-first century (Wheeler and Braun 2013). According to the simulation analysis, the average wheat yield decreased by 1–28% during 1981–2010 period caused by rising temperature (Asseng et al. 2015). Thus, the warming temperature causes severe wheat yield loss and imposes a substantial risk to global food security. To cope with climate variations and to protect themselves from injury and damage, wheat has evolved complex systems to improve their capability in response to heat stress. Therefore, understanding the molecular and genetic basis of the wheat response to heat stress would be helpful to develop new strategies to minimize deleterious impacts of heat stress during wheat breeding programs.
Phenotypic variation in reproductive stage responsive to heat stress in wheat
Heat stress imposes diverse negative effects on agronomic traits at different wheat developmental stages, but pre-flowering and anthesis stages are expected to be the most sensitive stages to heat stress (Cossani and Reynolds 2012), since unexpected high temperature could reduce pollen viability and subsequently decrease grain number, grain filling and grain quality (Asseng et al. 2011; Ugarte et al. 2007). It is reported that wheat pollen viability and seed setting rate will decrease significantly when the high temperature (> 30 °C) appears at the anthesis stage (Browne et al. 2021; Djanaguiraman et al. 2020). Consistently, a five-day period with moderate high temperate (~ 24 °C) at beginning of the heading period can reduce floret fertility by 15%, whereas extreme high temperature (~ 35 °C) will lead to complete abortion (Prasad and Djanaguiraman 2014). Not surprisingly, daytime high temperature (34 °C) at the anthesis stage significantly decreased wheat seed set from 7 to 19% (Sun et al. 2018). In addition, nighttime high temperature possesses similar effects to seed set rate, and 7-day-long high temperature at night (24 °C) in anthesis period result in decreased seed set by 15% in wheat (Narayanan et al. 2015).
Besides grain number, seed size and thousand kernel weight were also adversely affected by heat stress. Although high temperature can accelerate grain filling rate to some extent (Asseng et al. 2015; Barlow et al. 2015; Lobell et al. 2012), it shortens grain filling duration by 0.30–0.60% for every unit increase of high-temperature days when temperature exceed 30 °C (Liu et al. 2016). Bella and their colleagues reported that the duration and the timing of heat stress can explain 51.6% of phenotypic variation of thousand-kernel weight by analyzing more than 100 wheat varieties with varied geographic origins (Balla et al. 2019). Wang et al. (2018) found that late sowing can cause an increase of ~ 2 °C during the wheat filling stage and reduced the grain filling duration by 1–2 weeks, finally resulted in a substantial yield decrease. Bheemanahalli et al. (2019) examined daytime heat response of 28 spring wheat varieties during flowering and grain filling stage, and found ~ 32 and ~ 16% decrease of thousand kernel weight of main spike, respectively. Similarly, nighttime high temperature at post-anthesis stage also reduced wheat thousand-grain weight by ~ 3% per °C increase (García et al. 2016). Moreover, other studies confirmed these observations both in field and in greenhouse (Liu et al. 2020; Talukder et al. 2014a).
As we know, starch contributes about ~ 80% of the dry weight of wheat seed, which has a close link with wheat grain yield. Liu et al. (2011) applied 3-day period heat stress to wheat at the different filling stage from 1 to 33 days after flowering, and found different effects of heat stress at different periods of grain filling on grain starch formation of wheat. The effect of heat treatment at an early stage (6–8 days after flowering) is greater than that at late stage (36–38 days after flowering). Further investigation showed heat stress reduced both amylose and amylopectin concentration, yet amylopectin accumulation is more sensitive to the stress than that of amylose (Liu et al. 2011). Consistent with the observation, the expression patterns of starch biosynthesis-related genes changed seriously in response to heat stress, e.g. ADP-glucose pyrophosphorylase, one of the key enzymes during starch biosynthesis, was down-regulated after heat stress together with other related genes, and directly associated with the decrease of starch accumulation (Hurkman et al. 2003).
Genetic basis in response to heat stress in wheat
Heat stress tolerance is a quantitative trait contributed by many minor QTLs (Bohnert et al. 2006), and it is more difficult to measure phenotypic variation in response to heat stress compared with other agronomic traits. Therefore, there is very limited available information about the genetic basis of heat stress response in wheat, and none heat-tolerance gene was isolated according to map-based cloning strategy by now. Yet, many studies have been trying to map genetic loci controlling heat stress tolerance in wheat. In 1990’s, Sun and Quick reported that chromosomes 3A, 3B, 4A, 4B and 5A contained heat stress-tolerance related loci in tetraploid wheat because their corresponding chromosome substitution lines showed impaired heat tolerance by measuring membrane thermal stability (Sun and Quick 1991). Later, Sun’s group further confirmed the observation and found chromosomes 3A and 3B associated with heat tolerance in wheat cultivar Hope (Xu et al. 1996). In the twenty-first century, increasing heat stress-tolerance related QTL loci were reported taking advantage of developing molecular marker technology. Yang and the colleagues generated an F2 population including 166 individuals using heat-tolerant cultivar Ventnor and heat-susceptible cultivar Karl92, and identified two QTLs controlling grain-filling duration in response to heat stress on chromosomal 1B and 5A, which linked to the simple sequence repeat marker Xgwm11 and Xgwm293, respectively (Yang et al. 2002). Using a similar heat treatment to Yang’s method, Mohammadi et al. (2008) detected three heat-tolerance QTLs on chromosomes 1B, 5B and 7B in terms of heat susceptibility index (HSI, an indicator of heat response) by examining 144 recombinant inbred lines (RILs) with varied heat sensitivities derived from Kauz and MTRWA116 cultivar. Later, Mason et al. (2010) analyzed the HSI of yield component of a Halberd (heat tolerant)/Cutter (heat susceptible) RIL population under controlled heat stress environments (38 °C day/18 °C night), and detected 27 QTLs associated with improved heat tolerance, and among which, five (located on chromosomes 1A, 2A, 2B and 3B) were simultaneously detected in two-year experiments. Moreover, a follow-up study by the same group mapped 14 QTLs contributing to heat tolerance in wheat by calculating HSI of kernel number, total kernel weight, and single kernel weight coupled with temperature depression of the main spike and main flag leaf. Of these genomic loci, seven regions were consistently detected in their two continuous studies. Each QTL explains approximately 4.5–19.3% phenotypic variance, and a combination of the superior haplotype of three QTLs on chromosomes 1B, 5A, and 6D can improve the genetic effect of heat tolerance compared with a single locus (Mason et al. 2011). Pinto et al. (2010) also identified 16 QTLs associated with heat stress adaptive traits using Seri/Babax RIL population, and a QTL located on 4A explained 17% phenotypic variation under heat stress conditions. Interestingly, six common QTLs were found to contribute to both heat and drought stress tolerance, indicating a crosstalk between two stresses (Pinto et al. 2010). Paliwal et al. (2012) identified two heat tolerance QTLs on chromosomes 2B and 7B by analyzing HSI of 1000-grain weight, grain fill duration and canopy temperature of 144 wheat RIL lines, which explained phenotypic variation ranging from 9.78 to 20.34%. Sangwan et al. (2019) created a RIL population of wheat (Triticum aestivum L.) with heat-tolerant parent WH1021 and heat-sensitive parent WH711, significant genomic regions associated with heat tolerance were detected on chromosomes 2A, 2D, 4A and 5A, and a consistent QTL was found on chromosome 2D based on photosynthetic rate analysis. Zhai et al. (2021) located a TaHST1 locus in an interval of 0.949 Mbp at the distal terminus of 4AL chromosome arm, which contained 19 high confidence genes and contributed to both vegetative and reproductive growth of wheat under heat stress conditions. Moreover, genome-wide association analysis (GWAS) was also exploited to detect heat responsive QTLs using 205 wheat varieties with a late sown method, and a total of 69 potential QTLs were identified for ten different traits including grain filling duration and grain filling rate (Kumar et al. 2020). In addition, Wang et al. (2021) performed GWAS analysis of 688 diverse winter wheat accessions on thousand-grain weight and stress susceptibility index in response to heat stress using 90 K array, and revealed that terminal heat stress tolerance is not improved concurrently with grain weight during wheat breeding programs during recent decades, the authors proved superior alleles regulating both grain weight and heat tolerance, which can be used in marker-assisted selection for wheat in future. We summarized the reported QTLs-related heat response in wheat in Table 1 and Table S1.
Omics-based identification of heat-responsive genes in wheat
Since map-based cloning of the heat tolerance gene of wheat is still difficult in a forward genetic way, reverse genetic methods have been widely used to identify heat-responsive genes in wheat, e.g. multi-omics. Transcriptome analysis including microarray and RNA-seq is recognized as a high-throughput way to detect differentially expressed genes in response to heat stress. Qin and colleagues found that 10.7% probe sets were differentially expressed in response to 40 °C treatment at wheat seedling stage according to microarray analysis, which were involved in phytohormone biosynthesis, calcium and sugar signaling and ribosomal proteins related functional pathways (Qin et al. 2008). Later, Kumar et al. (2015a) identified 1525 heat-responsive genes using RNA-seq analysis, and reported that heat stress disturbed metabolic processes and oxidations-reductions processes in wheat. Moreover, as a typical allohexaploid, bread wheat experienced two independent hybridization and polyploidization events and theoretically contains three homeologs at each genomic loci. Liu et al.’s study revealed thousands of differentially expressed genes under heat stress conditions which exhibited varied time-course expression patterns. Interestingly, ~ 68.4% of homoeologous triplets showed diverse responses to heat stress, which might contribute to enhance thermotolerance in polyploid wheat (Liu et al. 2015).
Besides the transcriptional responses, post-transcriptional regulation also plays an important role in re-organizing transcriptome plasticity and proteomic complexity in response to heat stress. For example, alternative splicing (AS) refers to a RNA processing that multiple transcripts generate from a single gene, which extensively occurs in wheat genome (Yu et al. 2020). Liu and colleagues found that AS occurrence is increased by ~ 40% under heat stress conditions compared to normal conditions, and identified 3576 genes exhibiting AS changes in response to heat stress. It is worth noticing that a subset of homeologous triplets (7.5%) showed altered splicing patterns (Liu et al. 2015, 2018).
In addition, epigenetic modification is also involved in the post-transcriptional regulation of heat response in wheat including DNA methylation and non-coding RNAs. High temperature has a small but significant effect on gene methylation, and approximately 0.1% of genomic loci showed differential DNA methylation in wheat seedlings between 27 and 12 °C conditions. Of these sites, 63% of regions were also differentially expressed in response to elevated temperature, indicating differential methylation is closely associated with expression changes in wheat (Gardiner et al. 2015). Moreover, non-coding RNAs are also reported to participate in regulating heat response in wheat (Kumar et al. 2015b; Ragupathy et al. 2016; Xin et al. 2010). For example, TamiR159 was downregulated after 2 h heat treatment in heat-sensitive wheat genotype, which targets TaGAMYB1 and TaGAMYB2 and directs their cleavage. Overexpression of TamiR159 in rice caused increased heat sensitivity compared with wild type (Wang et al. 2012). In addition, Xin et al. identified 77 differentially expressed long non-coding RNAs before and after heat stress, parts of which functions probably by generating siRNAs, and interestingly, H3K9 acetylation is likely associated with long non-coding RNA expression patterns when subjecting to heat stress (Xin et al. 2011).
Wheat responses to heat stress also occur at the translational level. Pioneering studies discovered a set of proteins showing a changed abundance in response to heat stress using two-dimensional electrophoresis and MALDI-TOF–MS methods (Laino et al. 2010; Majoul et al. 2003, 2004; Yang et al. 2011). For example, it is reported that more low molecular weight proteins were produced in the flag leaf of heat–susceptible wheat cultivar than that of heat-tolerant cultivar in response to heat stress (Nandha et al. 2018). Whereas the abundance of proteins in flag leaf related to chlorophyll synthesis, carbon fixation, protein turnover and redox regulation were significantly altered at the grain filling stage (Lu et al. 2017). Furthermore, iTRAQ investigation identified 256 proteins showing differential expression patterns including 126 up-regulated and 130 down-regulated proteins. These proteins were enriched in stimulus response, stress response, kinase activity, and transferase activity categories (Zhang et al. 2017).
Functional genes in response to heat stress in wheat
Multi-omics studies provide lots of potential candidate genes responsible for heat tolerance, and their molecular functional and signaling pathway analyses further help us to understand underlying mechanisms. Heat shock proteins (HSPs), acting as molecular chaperones assisting correct protein conformation, were induced rapidly in transcriptome analysis under heat stress conditions mostly controlled by heat shock factors. Because the stress can lead to the accumulation of misfolded proteins, and HSPs would help these proteins with correct folding (Vierling 1991). Rampino et al. (2009) reported that the accumulation of HSP transcriptional abundance is proportional to the heat stress duration in durum wheat varieties, and contribute to acquired themo-tolerance. Wheat TaHSP23.9 was identified as a heat-responsive gene located in the endoplasmic reticulum based on TMT-labeled quantitative proteomic analysis, and its overexpression transgenic Arabidopsis exhibited improved heat tolerance (Wang et al. 2020). Heat shock factors (HSFs) also play a central role regulating HSP expression. There are 56 HSF transcription factors in wheat according to the previous prediction, and A2 and A6 type HSF members were highly induced upon heat stress (Xue et al. 2014). Consistently, Bi et al. (2020) demonstrated that ectopic expression of wheat TaHsfA6f in Arabidopsis resulted in improved tolerance to heat and other abiotic stresses in terms of seedling survival rate (Bi et al. 2020).
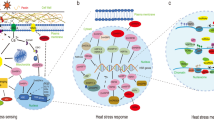
According to the transcriptome analysis, Geng et al. found that TabZIP60 was up-regulated and subjected to atypical alternative splicing after heat stress, depending on IRE1 gene which recognizes a dual stem-loop structure. Surprisingly, overexpression of heat-induced splicing form of wheat TabZIP60 (TabZIP60s) improved heat tolerance in Arabidopsis, but not for the unspliced form. As a transcription factor, TabZIP60s regulates expression patterns of 1104 genes in response to heat stress, including 35 genes, which significantly enriched in ER stress-related GO categories (Geng et al. 2018). In addition, Zang et al. found that TaFER (ferritin protein), TaPEPKR2 (phosphoenolpyruvate carboxylase kinase-related kinase protein), and TaOEP16-2 (plastid outer envelope protein) identified from heat stress-responsive transcriptome analysis, contributing to heat tolerance by overexpression analysis in Arabidopsis, and ROS accumulation is likely associated with heat tolerance in TaFER overexpression plants (Zang et al. 2017a, b, 2018). Further investigation revealed that constitutive expression of TaPEPKR2 in wheat resulted in enhanced tolerance to both heat and dehydration stresses (Zang et al. 2018). Interestingly, the chromosomal location of this gene is close to the genomic interval of heat tolerance-related QTL.ICD.Heat.09§ was identified by Hassouni et al. (2019) with a physical distance of ~ 2.7 Mb (Table S2). Guo et al. (2015) reported that overexpressing wheat NAC transcription factor TaNAC2L in Arabidopsis led to an increased survival rate of seedlings under heat stress conditions, and 26S proteasome is involved in the regulation of TaNAC2L protein abundance at post-transcriptional level in response to heat stress. Moreover, wheat 12-oxo-phytodienoic acid reductase (TaOPR3), involved in jasmonate (JA) biosynthesis, is up-regulated when facing heat stress, and its knockdown lines show enhanced heat sensitivity, whereas overexpression lines exhibit improved heat tolerance. In Arabidopsis, HSFA1b binds heat shock elements of AtOPR3, a homolog of TaOPR3, results in activation of AtOPR3 and JA accumulation after heat stress, indicating a mechanistic link between HSFs and JA signaling pathway in response to heat stress (Tian et al. 2020) (Fig. 1).
Conclusions
Heat stress is a limiting factor resulting in wheat yield loss worldwide, and the occurrence of heat events is projected to increase in the future. It is estimated that yield loss and post-heading heat stress are significantly correlated, especially, when heat stress occurred together with drought stress, their interaction will highlight yield variability, explaining approximately a third (32–39%) of wheat yield loss (Ray et al. 2015). Therefore, understanding the genetic basis and molecular mechanisms of heat response will pave a way to improve heat tolerance during wheat breeding programs. Yet, this quantitative agronomic trait is controlled by multiple genes with minor effects, and probably due to huge genomic constitution, no major gene responsive to heat stress has been isolated using map-based cloning method in wheat till now, although a bunch of heat stress-related QTLs were obtained. However, with the release of wheat reference genome and the advent of state-of-art technology, map-based gene cloning is becoming easier nowadays than before in wheat. Thus, it needs more effort to go into the project of heat stress gene cloning during subsequent studies. In addition, functional analysis of heat-responsive wheat gene is often performed in model plants in previous studies, because wheat transgene technology is not reliable then. However, the situation is changed now and overexpression, RNAi and CRISPR-Cas9 technology have been widely used in wheat recently. Therefore, we propose that map-based gene cloning and molecular mechanisms of heat response gene will speed up in wheat in the future. However, we have to notice that overexpression or pyramiding of heat-responsive gene often results in side effects on crop yield according to the previous studies in model plants. How to improve wheat heat tolerance without yield penalty is an important issue we have to face. The study of the rice TT1 gene provides us a new insight into the usage of heat-tolerant gene that the substitution of one amino acid might lead to protein conformation variation or protein stability change when subjected to heat stress, and subsequently contribute to heat tolerance (Li et al. 2015). Therefore, we should pay more attention to superior allele identification, which can both promote heat tolerance and reduce yield and quality penalty in a wheat breeding program.
References
Asseng S, Foster IA, Turner NC (2011) The impact of temperature variability on wheat yields. Glob Change Biol 17:997–1012. https://doi.org/10.1111/j.1365-2486.2010.02262.x
Asseng S, Ewert F, Martre P, Rötter RP, Lobell DB, Cammarano D, Kimball BA, Ottman MJ, Wall GW, White JW, Reynolds MP, Alderman PD, Prasad PVV, Aggarwal PK, Anothai J, Basso B, Biernath C, Challinor AJ, de Sanctis G, Doltra J, Fereres E, Garcia-Vila M, Gayler S, Hoogenboom G, Hunt LA, Izaurralde RC, Jabloun M, Jones CD, Kersebaum KC, Koehler A-K, Müller C, Naresh Kumar S, Nendel C, O’Leary G, Olesen JE, Palosuo T, Priesack E, Eyshi Rezaei E, Ruane AC, Semenov MA, Shcherbak I, Stöckle C, Stratonovitch P, Streck T, Supit I, Tao F, Thorburn PJ, Waha K, Wang E, Wallach D, Wolf J, Zhao Z, Zhu Y (2015) Rising temperatures reduce global wheat production. Nat Clim Change 5:143–147. https://doi.org/10.1038/nclimate2470
Balla K, Karsai I, Bónis P, Kiss T, Berki Z, Horváth Á, Mayer M, Bencze S, Veisz O (2019) Heat stress responses in a large set of winter wheat cultivars (Triticum aestivum L.) depend on the timing and duration of stress. PLoS ONE 14:e0222639. https://doi.org/10.1371/journal.pone.0222639
Barlow KM, Christy BP, O’Leary GJ, Riffkin PA, Nuttall JG (2015) Simulating the impact of extreme heat and frost events on wheat crop production: a review. Field Crop Res 171:109–119. https://doi.org/10.1016/j.fcr.2014.11.010
Bheemanahalli R, Sunoj VSJ, Saripalli G, Prasad PVV, Balyan HS, Gupta PK, Grant N, Gill KS, Jagadish SVK (2019) Quantifying the impact of heat stress on pollen germination, seed set, and grain filling in spring wheat. Crop Sci 59:684–696. https://doi.org/10.2135/cropsci2018.05.0292
Bhusal N, Sarial AK, Sharma P, Sareen S (2017) Mapping QTLs for grain yield components in wheat under heat stress. PLoS ONE 12:e0189594. https://doi.org/10.1371/journal.pone.0189594
Bi H, Zhao Y, Li H, Liu W (2020) Wheat heat shock factor TaHsfA6f increases ABA levels and enhances tolerance to multiple abiotic stresses in transgenic plants. Int J Mol Sci. https://doi.org/10.3390/ijms21093121
Bohnert HJ, Gong Q, Li P, Ma S (2006) Unraveling abiotic stress tolerance mechanisms—getting genomics going. Curr Opin Plant Biol 9:180–188. https://doi.org/10.1016/j.pbi.2006.01.003
Browne RG, Li SF, Iacuone S, Dolferus R, Parish RW (2021) Differential responses of anthers of stress tolerant and sensitive wheat cultivars to high temperature stress. Planta 254:4. https://doi.org/10.1007/s00425-021-03656-7
Cossani CM, Reynolds MP (2012) Physiological traits for improving heat tolerance in wheat. Plant Physiol 160:1710–1718. https://doi.org/10.1104/pp.112.207753
Djanaguiraman M, Narayanan S, Erdayani E, Prasad PVV (2020) Effects of high temperature stress during anthesis and grain filling periods on photosynthesis, lipids and grain yield in wheat. BMC Plant Biol 20:268. https://doi.org/10.1186/s12870-020-02479-0
Farooq M, Bramley H, Palta JA, Siddique KH (2011) Heat stress in wheat during reproductive and grain-filling phases. Crit Rev Plant Sci 30:491–507. https://doi.org/10.1080/07352689.2011.615687
García GA, Serrago RA, Dreccer MF, Miralles DJ (2016) Post-anthesis warm nights reduce grain weight in field-grown wheat and barley. Field Crop Res 195:50–59. https://doi.org/10.1016/j.fcr.2016.06.002
Gardiner L-J, Quinton-Tulloch M, Olohan L, Price J, Hall N, Hall A (2015) A genome-wide survey of DNA methylation in hexaploid wheat. Genome Biol 16:273. https://doi.org/10.1186/s13059-015-0838-3
Geng X, Zang X, Li H, Liu Z, Zhao A, Liu J, Peng H, Yao Y, Hu Z, Ni Z, Sun Q, Xin M (2018) Unconventional splicing of wheat TabZIP60 confers heat tolerance in transgenic Arabidopsis. Plant Sci 274:252–260. https://doi.org/10.1016/j.plantsci.2018.05.029
Guan P, Lu L, Jia L, Kabir MR, Zhang J, Lan T, Zhao Y, Xin M, Hu Z, Yao Y, Ni Z, Sun Q, Peng H (2018) Global QTL analysis identifies genomic regions on chromosomes 4A and 4B harboring stable loci for yield-related traits across different environments in wheat (Triticum aestivum L). Front Plant Sci 9:529. https://doi.org/10.3389/fpls.2018.00529
Guo W, Zhang J, Zhang N, Xin M, Peng H, Hu Z, Ni Z, Du J (2015) The wheat NAC transcription factor TaNAC2L Is regulated at the transcriptional and post-translational levels and promotes heat stress tolerance in transgenic Arabidopsis. PLoS ONE 10:e0135667. https://doi.org/10.1371/journal.pone.0135667
Hassan FSC, Solouki M, Fakheri BA, Nezhad NM, Masoudi B (2018) Mapping QTLs for physiological and biochemical traits related to grain yield under control and terminal heat stress conditions in bread wheat (Triticum aestivum L.). Physiol Mol Biol Plants 24:1231–1243. https://doi.org/10.1007/s12298-018-0590-8
Hassouni KE, Belkadi B, Filali-Maltouf A, Tidiane-Sall A, Bassi FM (2019) Loci controlling adaptation to heat stress occurring at the reproductive stage in durum wheat. Agronomy 9:414. https://doi.org/10.3390/agronomy9080414
Hurkman WJ, McCue KF, Altenbach SB, Korn A, Tanaka CK, Kothari KM, Johnson EL, Bechtel DB, Wilson JD, Anderson OD, DuPont FM (2003) Effect of temperature on expression of genes encoding enzymes for starch biosynthesis in developing wheat endosperm. Plant Sci 164:873–881. https://doi.org/10.1016/S0168-9452(03)00076-1
IWGSC (2014) A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 345:1251788. https://doi.org/10.1126/science.1251788
Kumar RR, Goswami S, Sharma SK, Kala YK, Rai GK, Mishra DC, Grover M, Singh GP, Pathak H, Rai A, Chinnusamy V, Rai RD (2015a) Harnessing next generation sequencing in climate change: RNA-Seq analysis of heat stress-responsive genes in wheat (Triticum aestivum L.). OMICS 19:632–647. https://doi.org/10.1089/omi.2015.0097
Kumar RR, Pathak H, Sharma SK, Kala YK, Nirjal MK, Singh GP, Goswami S, Rai RD (2015b) Novel and conserved heat-responsive microRNAs in wheat (Triticum aestivum L.). Funct Integr Genomics 15:323–348. https://doi.org/10.1007/s10142-014-0421-0
Kumar S, Kumari J, Bhusal N, Pradhan AK, Budhlakoti N, Mishra DC, Chauhan D, Kumar S, Singh AK, Reynolds M, Singh GP, Singh K, Sareen S (2020) Genome-wide association study reveals genomic regions associated with ten agronomical traits in wheat under late-sown conditions. Front Plant Sci 11:549743. https://doi.org/10.3389/fpls.2020.549743
Laino P, Shelton D, Finnie C, de Leonardis AM, Mastrangelo AM, Svensson B, Lafiandra D, Masci S (2010) Comparative proteome analysis of metabolic proteins from seeds of durum wheat (cv. Svevo) subjected to heat stress. Proteomics 10:2359–2368. https://doi.org/10.1002/pmic.200900803
Li X-M, Chao D-Y, Wu Y, Huang X, Chen K, Cui L-G, Su L, Ye W-W, Chen H, Chen H-C, Dong N-Q, Guo T, Shi M, Feng Q, Zhang P, Han B, Shan J-X, Gao J-P, Lin H-X (2015) Natural alleles of a proteasome α2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat Genet 47:827–833. https://doi.org/10.1038/ng.3305
Li L, Mao X, Wang J, Chang X, Reynolds M, Jing R (2019) Genetic dissection of drought and heat-responsive agronomic traits in wheat. Plant Cell Environ 42:2540–2553. https://doi.org/10.1111/pce.13577
Liu P, Guo W, Jiang Z, Pu H, Feng C, Zhu X, Peng Y, Kuang A, Little CR (2011) Effects of high temperature after anthesis on starch granules in grains of wheat (Triticum aestivum L.). J Agric Sci 149:159–169. https://doi.org/10.1017/S0021859610001024
Liu Z, Xin M, Qin J, Peng H, Ni Z, Yao Y, Sun Q (2015) Temporal transcriptome profiling reveals expression partitioning of homeologous genes contributing to heat and drought acclimation in wheat (Triticum aestivum L). BMC Plant Biol 15:152. https://doi.org/10.1186/s12870-015-0511-8
Liu B, Asseng S, Liu L, Tang L, Cao W, Zhu Y (2016) Testing the responses of four wheat crop models to heat stress at anthesis and grain filling. Glob Change Biol 22:1890–1903. https://doi.org/10.1111/gcb.13212
Liu Z, Qin J, Tian X, Xu S, Wang Y, Li H, Wang X, Peng H, Yao Y, Hu Z, Ni Z, Xin M, Sun Q (2018) Global profiling of alternative splicing landscape responsive to drought, heat and their combination in wheat (Triticum aestivum L.). Plant Biotechnol J 16:714–726. https://doi.org/10.1111/pbi.12822
Liu B, Liu L, Asseng S, Zhang D, Ma W, Tang L, Cao W, Zhu Y (2020) Modelling the effects of post-heading heat stress on biomass partitioning, and grain number and weight of wheat. J Exp Bot 71:6015–6031. https://doi.org/10.1093/jxb/eraa310
Lobell DB, Sibley A, Ivan Ortiz-Monasterio J (2012) Extreme heat effects on wheat senescence in India. Nat Clim Change 2:186–189. https://doi.org/10.1038/nclimate1356
Lu Y, Li R, Wang R, Wang X, Zheng W, Sun Q, Tong S, Dai S, Xu S (2017) Comparative proteomic analysis of flag leaves reveals new insight into wheat heat adaptation. Front Plant Sci 8:1086. https://doi.org/10.3389/fpls.2017.01086
Majoul T, Bancel E, Triboï E, Ben Hamida J, Branlard G (2003) Proteomic analysis of the effect of heat stress on hexaploid wheat grain: characterization of heat-responsive proteins from total endosperm. Proteomics 3:175–183. https://doi.org/10.1002/pmic.200390026
Majoul T, Bancel E, Triboï E, Ben Hamida J, Branlard G (2004) Proteomic analysis of the effect of heat stress on hexaploid wheat grain: characterization of heat-responsive proteins from non-prolamins fraction. Proteomics 4:505–513. https://doi.org/10.1002/pmic.200300570
Mason RE, Mondal S, Beecher FW, Pacheco A, Jampala B, Ibrahim AMH, Hays DB (2010) QTL associated with heat susceptibility index in wheat (Triticum aestivum L.) under short-term reproductive stage heat stress. Euphytica 174:423–436. https://doi.org/10.1007/s10681-010-0151-x
Mason RE, Mondal S, Beecher FW, Hays DB (2011) Genetic loci linking improved heat tolerance in wheat (Triticum aestivum L.) to lower leaf and spike temperatures under controlled conditions. Euphytica 180:181–194. https://doi.org/10.1007/s10681-011-0349-6
Maulana F, Ayalew H, Anderson JD, Kumssa TT, Huang W, Ma X-F (2018) Genome-wide association mapping of seedling heat tolerance in winter wheat. Front Plant Sci 9:1272. https://doi.org/10.3389/fpls.2018.01272
Mohammadi V, Zali AA, Bihamta MR (2008) Mapping QTLs for heat tolerance in wheat. J Agric Sci Technol 10:261–267
Nandha AK, Mehta DR, Tulsani NJ, Umretiya N, Delvadiya N (2018) Proteomic analysis in wheat to study the effect of heat stress on flag leaf. Int J Curr Microbiol Appl Sci 7:3432–3439. https://doi.org/10.20546/ijcmas.2018.702.409
Narayanan S, Prasad PVV, Fritz AK, Boyle DL, Gill BS (2015) Impact of high night-time and high daytime temperature stress on winter wheat. J Agro Crop Sci 201:206–218. https://doi.org/10.1111/jac.12101
Paliwal R, Röder MS, Kumar U, Srivastava JP, Joshi AK (2012) QTL mapping of terminal heat tolerance in hexaploid wheat (T. aestivum L.). Theor Appl Genet 125:561–575. https://doi.org/10.1007/s00122-012-1853-3
Pinto RS, Reynolds MP, Mathews KL, McIntyre CL, Olivares-Villegas J-J, Chapman SC (2010) Heat and drought adaptive QTL in a wheat population designed to minimize confounding agronomic effects. Theor Appl Genet 121:1001–1021. https://doi.org/10.1007/s00122-010-1351-4
Prasad PVV, Djanaguiraman M (2014) Response of floret fertility and individual grain weight of wheat to high temperature stress: sensitive stages and thresholds for temperature and duration. Funct Plant Biol 41:1261–1269. https://doi.org/10.1071/FP14061
Qin D, Wu H, Peng H, Yao Y, Ni Z, Li Z, Zhou C, Sun Q (2008) Heat stress-responsive transcriptome analysis in heat susceptible and tolerant wheat (Triticum aestivum L) by using Wheat Genome Array. BMC Genom 9:432. https://doi.org/10.1186/1471-2164-9-432
Ragupathy R, Ravichandran S, Mahdi MSR, Huang D, Reimer E, Domaratzki M, Cloutier S (2016) Deep sequencing of wheat sRNA transcriptome reveals distinct temporal expression pattern of miRNAs in response to heat, light and UV. Sci Rep 6:39373. https://doi.org/10.1038/srep39373
Rampino P, Mita G, Pataleo S, de Pascali M, Di Fonzo N, Perrotta C (2009) Acquisition of thermotolerance and HSP gene expression in durum wheat (Triticum durum Desf.) cultivars. Environ Exp Bot 66:257–264. https://doi.org/10.1016/j.envexpbot.2009.04.001
Ray DK, Mueller ND, West PC, Foley JA (2013) Yield trends are insufficient to double global crop production by 2050. PLoS ONE 8:e66428. https://doi.org/10.1371/journal.pone.0066428
Ray DK, Gerber JS, MacDonald GK, West PC (2015) Climate variation explains a third of global crop yield variability. Nat Commun 6:5989. https://doi.org/10.1038/ncomms6989
Sangwan S, Munjal R, Ram K, Kumar N (2019) QTL mapping for morphological and physiological traits in RILs of spring wheat population of WH1021 × WH711. JEB 40:674–682. https://doi.org/10.22438/jeb/40/4/MRN-1002
Shirdelmoghanloo H, Taylor JD, Lohraseb I, Rabie H, Brien C, Timmins A, Martin P, Mather DE, Emebiri L, Collins NC (2016) A QTL on the short arm of wheat (Triticum aestivum L) chromosome 3B affects the stability of grain weight in plants exposed to a brief heat shock early in grain filling. BMC Plant Biol 16:100. https://doi.org/10.1186/s12870-016-0784-6
Sun QX, Quick JS (1991) Chromosomal locations of genes for heat tolerance in tetraploid wheat. Cereal Res Commun 19:431–437
Sun A, Impa S, Valiaparambil SS, Kanwardeep S, Kulvinder G, Prasad PVV, Krishna JSV (2018) Heat stress during flowering affects time of day of flowering, seed set, and grain quality in spring wheat. Crop Sci 58:380–392. https://doi.org/10.2135/cropsci2017.04.0221
Talukder A, McDonald GK, Gill GS (2014a) Effect of short-term heat stress prior to flowering and early grain set on the grain yield of wheat. Field Crop Res 160:54–63. https://doi.org/10.1016/j.fcr.2014.01.013
Talukder SK, Babar MA, Vijayalakshmi K, Poland J, Prasad P, Bowden R, Fritz A (2014b) Mapping QTL for the traits associated with heat tolerance in wheat (Triticum aestivum L.). BMC Genet 15:1–13. https://doi.org/10.1186/s12863-014-0097-4
Telfer P, Edwards J, Norman A, Bennett D, Smith A, Able JA, Kuchel H (2021) Genetic analysis of wheat (Triticum aestivum) adaptation to heat stress. Theor Appl Genet 134:1387–1407. https://doi.org/10.1007/s00122-021-03778-2
Tian X, Wang F, Zhao Y, Lan T, Yu K, Zhang L, Qin Z, Hu Z, Yao Y, Ni Z, Sun Q, Rossi V, Peng H, Xin M (2020) Heat shock transcription factor A1b regulates heat tolerance in wheat and Arabidopsis through OPR3 and jasmonate signalling pathway. Plant Biotechnol J 18:1109–1111. https://doi.org/10.1111/pbi.13268
Ugarte C, Calderini DF, Slafer GA (2007) Grain weight and grain number responsiveness to pre-anthesis temperature in wheat, barley and triticale. Field Crop Res 100:240–248. https://doi.org/10.1016/j.fcr.2006.07.010
Vierling E (1991) The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 42:579–620
Wang Y, Sun F, Cao H, Peng H, Ni Z, Sun Q, Yao Y (2012) TamiR159 directed wheat TaGAMYB cleavage and its involvement in another development and heat response. PLoS ONE 7:e48445. https://doi.org/10.1371/journal.pone.0048445
Wang X, Hou L, Lu Y, Wu B, Gong X, Liu M, Wang J, Sun Q, Vierling E, Xu S (2018) Metabolic adaptation of wheat grain contributes to a stable filling rate under heat stress. J Exp Bot 69:5531–5545. https://doi.org/10.1093/jxb/ery303
Wang J, Gao X, Dong J, Tian X, Wang J, Palta JA, Xu S, Fang Y, Wang Z (2020) Over-expression of the heat-responsive wheat gene TaHSP23.9 in transgenic arabidopsis conferred tolerance to heat and salt stress. Front Plant Sci 11:243. https://doi.org/10.3389/fpls.2020.00243
Wang X, Guan P, Xin M, Wang Y, Chen X, Zhao A, Liu M, Li H, Zhang M, Lu L, Zhang J, Ni Z, Yao Y, Hu Z, Peng H, Sun Q (2021) Genome-wide association study identifies QTL for thousand grain weight in winter wheat under normal- and late-sown stressed environments. Theor Appl Genet 134:143–157. https://doi.org/10.1007/s00122-020-03687-w
Wheeler T, von Braun J (2013) Climate change impacts on global food security. Science 341:508–513. https://doi.org/10.1126/science.1239402
Xin M, Yu W, Yao Y, Xie C, Peng H, Ni Z, Sun Q (2010) Diverse set of microRNAs are responsive to powdery mildew infection and heat stress in wheat (Triticum aestivum L). BMC Plant Biol 10:123. https://doi.org/10.1186/1471-2229-10-123
Xin M, Wang Y, Yao Y, Song N, Hu Z, Qin D, Xie C, Peng H, Ni Z, Sun Q (2011) Identification and characterization of wheat long non-protein coding RNAs responsive to powdery mildew infection and heat stress by using microarray analysis and SBS sequencing. BMC Plant Biol 11:61. https://doi.org/10.1186/1471-2229-11-61
Xu R, Sun Q, Zhang S (1996) Chromosomal location of genes for heat tolerance as measured by membrane thermostability of common wheat cv Hope. Yi Chuan 18:1–3
Xue G-P, Sadat S, Drenth J, McIntyre CL (2014) The heat shock factor family from Triticum aestivum in response to heat and other major abiotic stresses and their role in regulation of heat shock protein genes. J Exp Bot 65:539–557. https://doi.org/10.1093/jxb/ert399
Yang J, Sears RG, Gill BS, Paulsen GM (2002) Quantitative and molecular characterization of heat tolerance in hexaploid wheat. Euphytica 126:275–282. https://doi.org/10.1023/A:1016350509689
Yang F, Jørgensen AD, Li H, Søndergaard I, Finnie C, Svensson B, Jiang D, Wollenweber B, Jacobsen S (2011) Implications of high-temperature events and water deficits on protein profiles in wheat (Triticum aestivum L. cv. Vinjett) grain. Proteomics 11:1684–1695. https://doi.org/10.1002/pmic.201000654
Yu K, Feng M, Yang G, Sun L, Qin Z, Cao J, Wen J, Li H, Zhou Y, Chen X, Peng H, Yao Y, Hu Z, Guo W, Sun Q, Ni Z, Adams K, Xin M (2020) Changes in alternative splicing in response to domestication and polyploidization in wheat. Plant Physiol 184:1955–1968. https://doi.org/10.1104/pp.20.00773
Zang X, Geng X, Liu K, Wang F, Liu Z, Zhang L, Zhao Y, Tian X, Hu Z, Yao Y, Ni Z, Xin M, Sun Q, Peng H (2017a) Ectopic expression of TaOEP16-2-5B, a wheat plastid outer envelope protein gene, enhances heat and drought stress tolerance in transgenic Arabidopsis plants. Plant Sci 258:1–11. https://doi.org/10.1016/j.plantsci.2017.01.011
Zang X, Geng X, Wang F, Liu Z, Zhang L, Zhao Y, Tian X, Ni Z, Yao Y, Xin M, Hu Z, Sun Q, Peng H (2017b) Overexpression of wheat ferritin gene TaFER-5B enhances tolerance to heat stress and other abiotic stresses associated with the ROS scavenging. BMC Plant Biol 17:14. https://doi.org/10.1186/s12870-016-0958-2
Zang X, Geng X, He K, Wang F, Tian X, Xin M, Yao Y, Hu Z, Ni Z, Sun Q, Peng H (2018) Overexpression of the wheat (Triticum aestivum L) TaPEPKR2 gene enhances heat and dehydration tolerance in both wheat and Arabidopsis. Front Plant Sci 9:1710. https://doi.org/10.3389/fpls.2018.01710
Zhai H, Jiang C, Zhao Y, Yang S, Li Y, Yan K, Wu S, Luo B, Du Y, Jin H, Liu X, Zhang Y, Lu F, Reynolds M, Ou X, Qiao W, Jiang Z, Peng T, Gao D, Hu W, Wang J, Gao H, Yin G, Zhang K, Li G, Wang D (2021) Wheat heat tolerance is impaired by heightened deletions in the distal end of 4AL chromosomal arm. Plant Biotechnol J 19:1038–1051. https://doi.org/10.1111/pbi.13529
Zhang Y, Pan J, Huang X, Guo D, Lou H, Hou Z, Su M, Liang R, Xie C, You M, Li B (2017) Differential effects of a post-anthesis heat stress on wheat (Triticum aestivum L) grain proteome determined by iTRAQ. Sci Rep 7:3468. https://doi.org/10.1038/s41598-017-03860-0
Author information
Authors and Affiliations
Contributions
We apologize to colleagues whose work is not mentioned here due to space limitation. The study was supported by Major Program of the National Natural Science Foundation of China (3213000343).
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, L., Wen, J., Peng, H. et al. The genetic and molecular basis for improving heat stress tolerance in wheat. aBIOTECH 3, 25–39 (2022). https://doi.org/10.1007/s42994-021-00064-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42994-021-00064-z