Abstract
The photo-identification of uniquely marked individuals has revealed much about mammalian behaviour and social structure in recent decades. In bottlenose dolphins (Tursiops spp.), for example, the long-term tracking of individuals has unveiled considerable variation in social structure among populations and various spatio-temporal aspects of group formation. In this study, we investigated associations among individual males in a small community of Indo-Pacific bottlenose dolphins (T. aduncus) residing in an urbanized estuary in southwestern Australia. Given the relative proximity of our study area to other populations in which complex male alliances form for the purpose of mate acquisition, we used long-term photo-identification records and social analyses to assess whether such alliances also occur in smaller and more isolated settings. Our work revealed strong social bonds and long-term, non-random associations among individual males, suggesting the occurrence of male alliances. Behavioural observations of alliances interacting with potentially receptive adult females from the estuary community and from adjacent communities, and exhibiting sexual display behaviours near females, suggest that these alliances occur in a reproductive context. As the first formal analysis indicating the occurrence of male alliances outside Shark Bay along the vast western coastline of Australia, this study complements previous research and extends our understanding of the evolutionary and ecological processes that drive alliance formation.
Similar content being viewed by others
Introduction
Photo-identification is a widely used and largely non-invasive tool that forms an integral component of many field studies in conservation biology. This technique uses natural marks recorded in images of both terrestrial and marine taxa to recognize individual animals with idiosyncratic fur and coat patterns (e.g., zebras Equus burchelli, Petersen 1972; monk seals Monachus monachus, Forcada and Aguilar 2000; giraffes Giraffa camelopardalis, Le Pendu et al. 2000); pigmentation and spots (e.g., whale sharks Rhincodon typus, Meekan et al. 2006); and scars or other marks (e.g., nicks and notches on the flukes or dorsal fins of cetaceans, Würsig and Jefferson 1990). It has proven a remarkably useful approach in understanding life history patterns (Hammond et al. 1990) and allowing mark-recapture studies to assess abundance and apparent survival (e.g., Lebreton et al. 1992; Meekan et al. 2006; Chabanne et al. 2017b). When combined with spatial and temporal information, photo-identification can be used to assess traits such as density, sex, site fidelity, movement patterns and home range size (Brown et al. 2012; Chabanne et al. 2012; Brown et al. 2016a, b).
Photo-identification has also been combined with behavioural sampling and other quantitative methods to investigate social structure (e.g., Connor et al. 1992a, b; Le Pendu et al. 2000). Indeed, these techniques have enabled researchers to elucidate the variation in, and complexity of, phenomena such as male alliance formation in various delphinids, including bottlenose dolphins (Tursiops spp.), across a broad range of habitat types and population densities (Table A1). To date, male alliances have been documented in common bottlenose (Tursiops truncatus), Risso’s (Grampus griseus), Australian humpback (Sousa sahulensis) and, perhaps most notably, Indo-Pacific bottlenose dolphins (T. aduncus, Owen et al. 2002; Hartman et al. 2008; Connor and Krützen 2015; Allen et al. 2017). Among coastal bottlenose dolphin populations, alliance formation spans the spectrum from no alliances (e.g., Baker et al. 2019), through a first level of alliance consisting of 2–4 closely-bonded individuals (e.g., Wells et al. 1987; Wiszniewski et al. 2012a, b), to the nested, multi-level alliance system found in Shark Bay, Western Australia (Connor et al. 1992a, b, 1999; Table A1). In Shark Bay, ‘first-order’ alliances consist of pairs or trios that cooperate in herding single oestrus females, and these pairs or trios are members of stable teams of 4–14 males at the ‘second-order’ level (Connor and Krützen 2015). Second-order alliances, the core social unit of males in the population, cooperate to attack other alliances for access to females, and to defend against such attacks (Connor and Krützen 2015). Remarkably, a third level of alliance formation is evident, involving two or more second-order alliances supporting each other in the capture and defence of females from other alliances (Connor et al. 2011, 2019; Randić et al. 2012; King et al. 2021). These studies have demonstrated how photo-identification, coupled with rigorous observational sampling and quantitative analyses, can provide behaviourally meaningful data on taxa that spend much of their time undetectable to researchers (Whitehead and Dufault 1999; Whitehead 2008a, b).
Several bottlenose dolphin populations in different habitats around the expansive western coastline of Australia have been studied (Allen et al. 2012, 2016; Sprogis et al. 2015; Brown et al. 2016a, b; Chabanne et al. 2017a; Raudino et al. 2018; Haughey et al. 2020) but, to date, male alliance formation has only been quantified in Shark Bay (Connor et al. 1992a, b), or speculated to exist elsewhere (Sprogis et al. 2015). For this study, we focused on a small community of Indo-Pacific bottlenose dolphins inhabiting the Swan Canning Riverpark (SCR), an urbanized estuarine system located 800 km south of Shark Bay, in which photo-identification has been conducted seasonally and yearly since 2011.
Here, we used sighting histories of the resident adult dolphins to investigate the potential occurrence of male alliances. We tested home range overlap, relatedness, and gregariousness as variables that may explain male associations with multiple regression quadratic assignment procedures (MRQAP). We also examined male association patterns using hierarchical clustering analysis, lagged association rates (LARs) and permutation procedures. Given the stable associations between individuals of the same sex and the strong bonds between some males described previously (Chabanne et al. 2012, 2017a), we predicted that, despite the small community size, males residing in the SCR also form alliances. We discuss the findings in light of the challenges of studying mammalian social behaviour, including alliance formation, within small communities.
Materials and methods
Study site and data collection
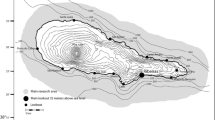
The Swan Canning Riverpark, Western Australia, is a 55 km2 micro-tidal estuary comprising two rivers running through the city of Perth, reaching the Indian Ocean through the Inner Harbour of the Port of Fremantle (Fig. 1). The SCR is home to a small community of around 16 adult Indo-Pacific bottlenose dolphins that are year-round residents, exhibiting long-term site fidelity (Chabanne et al. 2017a, b). However, the dolphin community is not isolated, with genetic and demographic exchange occurring with adjacent communities residing in a semi-enclosed embayment with large areas of shallow habitats (Fig. 1; Chabanne et al. 2021). Dolphin density in SCR is estimated to be 0.29 individuals per km2 (min: 0.18; max: 0.42; Chabanne et al. 2017b).
Photographic identification and behavioural data were collected during boat-based surveys conducted between June 2011 and March 2017 in the SCR and following the protocols described in Chabanne et al. (2012, 2017b). A group was defined as any individual engaging in the same behaviour and within 10 m of another (Smolker et al. 1992). Data collected for each encountered group included dolphin group size and composition, predominant behaviour recorded during the first 5 min of encountering the group (i.e., > 50% of individuals within a group were engaged in same behaviour [travel, forage, socialise, rest, or unknown], Mann 1999), location (GPS), and environmental conditions. We estimated and reviewed the age-class of the individuals during the study period as described in Chabanne et al. (2012). Individuals were sexed through molecular analyses of tissue samples (Chabanne et al. 2021) collected via remote biopsy sampling (Krützen et al. 2002).
Data restrictions for association patterns
We examined male associations using group sightings composed of at least one adult male and for which all individuals were identified with high-quality images (Chabanne et al. 2017b). Given the long-term dataset and survey frequency, we also used temporary marks for identification of individuals with non-distinct dorsal fins. We restricted our dataset to adult males that were alive over the entire course of the study period to account for any biases on the associations between individuals associated with demographic changes. Using SOCPROG 2.9 (Whitehead 2019), we checked for the accuracy of the social representation obtained with the restricted dataset by examining the social differentiation (S, measure of variability of the associations) and Pearson’s correlation coefficient (r, measure of the quality of the representation of the association pattern) following Whitehead (2008a, b). We set a daily sampling period and generated a matrix of association based on the Simple Ratio Index (SRI); the choice for using this index is justified by the high proportion of dolphins encountered being identified and the assumption that all associations were measured accurately being met (Cairns and Schwager 1987; Ginsberg and Young 1992).
We performed a multiple regression quadratic assignment procedure (MRQAP) with 1000 permutations to test the significance (2-tailed test with 0.05 p value) of three individual variables (home range overlap, relatedness, and gregariousness) on the SRIs while controlling for each other (Whitehead 2019). This preliminary test allowed the identification of individual variables that may exert undue influences on the true SRI values. We calculated home range overlap between each pair of adults based on a kernel-based utilization distribution overlap index (UDOI, Fieberg and Kochanny 2005) using the R package “Adehabitat” (Calenge 2006), followed by building a matrix using the UDOIs between each pair of males (see Fig. A1 for the 95% kernel density of each of the eight males). The matrix for gregariousness, a measure of the tendency of an individual to associate with other individuals (Godde et al. 2013), was created in SOCPROG following Whitehead and James (2015). Finally, we estimated relatedness of each pair of males using the TrioML estimator from the R package “related” (Pew et al. 2015) based on 10 microsatellite loci (Chabanne et al. 2021).
All association analyses were run using non-corrected SRIs. However, all pairwise association analyses including permutations tests were also carried out using Generalized Affiliations Indices (GAI), i.e., SRIs corrected for gregariousness (Whitehead and James 2015). In Appendix 3, we report the results using the GAIs, which did not differ from the analyses using the uncorrected SRIs.
Evaluation of male associations
We determined association strength for each male by comparing his maximum SRI with the mean SRI for all males. Associations were defined as ‘strong’ when their respective SRI values were at least twice the mean of all male dyads, and where each member of the male dyad ranked as each other’s closest associate (Wells et al. 1987; Connor et al. 1992a, b). We further produced a hierarchical average cluster analysis to visualize the degree of associations between males and calculated a cophenetic correlation coefficient (CCC), for which a CCC > 0.8 indicates a good representation of the hierarchical structure among individuals based on their association matrix (Bridge 1993). We also carried out a changepoint analysis using the Pruned Exact Linear Time (PELT) method in R 3.6.2 (R Development Core Team 2013), package ‘changepoint’ (Killick and Eckley 2014), to find the threshold values of SRIs characterizing multiple levels of strength among male dyads (Bizzozzero et al. 2019; Gerber et al. 2020).
We tested for the existence of long-term preferences between males by following the Monte-Carlo resampling (i.e., permutation test) procedure established by Whitehead et al. (2005). We considered the pattern significant when the coefficient of variation of the real association indices (SRI) was higher than expected by chance (Whitehead and James 2015; Whitehead 2019). We also extended the test to dyadic SRI values (Bejder et al. 1998) and reported ‘preferred’ associations when the observed number of significant dyads was larger than the expected (Whitehead 2019). Association estimates of a dyad at or above the 97.5 percentile were considered ‘preference’ (Whitehead et al. 2005; Whitehead 2019).
We also evaluated the long-term stability of associations by calculating Lagged Association Rates (LARs, Whitehead 1995) for males using real and random SRIs. The latter were available after we tested for preferred associations using a permutation test. They reflected the random distribution of associations without the temporal context (in comparison to the null association rate [NAR], which we also plotted). We obtained standard errors and precision estimates of LARs and NARs with a temporal jackknife procedure using a grouping factor of one day (Whitehead 2008a, b). The shapes of real and random LARs were compared to understand the implications of any changing patterns of associations over time (Whitehead 2019). This analysis was repeated with males having ‘strong’ bonds only (see results).
The small sample size (i.e., number of males) and our sampling method (i.e., a 5-min scan sample to determine predominant group activity and group size and composition, with ad libitum recording of some behavioural events) did not allow further exploration of the functional behaviour of the strongly bonded males as part of this study. Instead, we checked for any dependency between the presence of allied males and the presence of females and their residency area (Chabanne et al. 2017a) using Pearson’s chi-square statistics in the ‘Stats’ package in R (R Core Team 2013). Graphic mosaic plots were carried out using the ‘vcd’ package in R (Meyer et al. 2021).
Results
From June 2011 to March 2017, we conducted 187 surveys and tallied 304 dolphin group sightings, of which 250 group sightings were retained after excluding those with any unidentified or poorly photographed individuals. In our social analyses, we retained only sightings containing at least one adult male. A well-differentiated and accurate representation of the true social network was obtained when males were observed more than 12 times (r = 0.8 ± 0.04 SE; S = 0.9 ± 0.07 SE), leaving eight males in our dataset (Supplementary Material 1). Thus, patterns of male associations were assessed based on 175 group sightings. During the study period, no permanent emigration or deaths were recorded, meaning there was no demographic effect that could bias the males’ associations (Analysis of Lagged Identification Rate, Supplementary Material 2). Thirteen and eleven adult females residing in the SCR estuary or visiting from adjacent communities, respectively, were present in 61% of these sightings.
MRQAP tests indicated that neither relatedness nor home range overlap explained the strength of association between males. However, gregariousness did so, suggesting that the SRI was affected by gregariousness (Table 1).
Mean SRI between all adult male dyads was 0.25 (SD 0.05), with an average maximum SRI of 0.65 (SD 0.27, Table 2). The first changepoint occurred at 0.77, revealing a dyad (EXT/PRI) and a triad (ARR/BOT/HII) of males (Fig. 2), with all five males having a maximum SRI higher than the average maximum (Table 2). Such bond strengths would qualify these males as first-order alliances in Shark Bay and other populations (e.g., Connor and Krützen 2015; Ermak et al. 2017). A second changepoint at SRI ≥ 0.28, and above the mean SRI between all male dyads, resulted in two single males (PEB and KWL) being closely associated with the dyad (EXT/PRI, Fig. 2). A third changepoint was identified at 0.23. Since the value was lower than the mean SRI, the associations of BLA with the triad (ARR/BOT/HII) were not considered close under the criteria for male alliance formation (Fig. 2).
Dendrogram produced using hierarchical average cluster analysis with SRIs (CCC = 0.98033) of the eight male bottlenose dolphins (i.e., three-letter code) in the SCR. Levels of grouping obtained with the PELT changepoint method are displayed in dashed black vertical lines. Coloured solid lines denote the males forming strong bonds (SRI > 0.77 illustrating the dyad and triad), and the blue dashed lines denote the ‘close associates’ relationships (SRI > 0.28 − 0.77). Black solid lines are non-significant relationships under the criteria for male alliance formation
The permutation test for long-term preferred associations among males was significant (coefficient of variation CV real data = 1.067; CV random data = 0.856, p < 0.0001). Among the closest associates, we identified the triad ARR/BOT/HII and the dyad EXT/PRI as first-order allies (Table 3).
Both real and random LAR projections for first-order ally males were well above the NAR projection, supporting non-random association over the entire study period. In addition, the real LAR was higher than the random LAR, confirming the long-term preferred associations among the first-order allies that do not change over time (Fig. 3).
Lagged Association Rates from the real data (LARs; solid line) for all males (black) and for males of first-order alliances (blue) within the SCR resident dolphins. Their respective null association rates are indicated in dotted coloured lines of respective colour, and their LARs from the random data generated from the permutation test are in dashed coloured lines. Jackknife error bars are shown as the vertical lines
Triad formation (Appendix Fig. A3) among our first-order alliance members (ARR/BOT/HII) was significantly lower in the absence of females but highly dependent upon the presence of females from adjacent waters (X2 = 53.311, df = 4, p < 0.001, Fig. 4), with these females never seen in the SCR without the resident allied males.
On two occasions, we observed two males (BOT and BLA) performing a ‘rooster strut’, i.e., a sexual display performed by individual males in the presence of oestrus females, during which the male bobs his head up and down at the water surface while moving forward (Connor et al. 2000, Appendix Fig. A4).
Discussion
This study aimed to identify the occurrence of male alliances in a small community of Indo-Pacific bottlenose dolphins residing in a south-western Australian estuary. Using photo-identification data of resident dolphins in the Swan Canning River Park from 2011 to 2017, we inferred the occurrence of male alliances, based on three lines of evidence. First, our detailed quantitative analysis clearly indicated the presence of closely bonded adult males who associate over a long temporal scale. Second, we invariably observed non-resident females, in some cases as much as 20 km outside their normal ranges, in the same group as the allied triad. This is suggestive of these females having been herded outside their normal ranges, as previously documented in Shark Bay (Connor et al. 1996; Scott et al. 2005; Tsai and Mann 2013). Third, we documented opportunistic behavioural observations, such as the rooster struts performed by adult males around oestrus females (Connor et al. 2000). All three lines of evidence suggest the presence of male alliances linked to reproductive purposes, as observed elsewhere (Krutzen et al. 2004; Wiszniewski et al. 2012a, b).
Social bond strength among males was not influenced by their relatedness or home range overlap, but by differences in gregariousness, which is common in animal populations (Godde et al. 2013). All SCR residents share the same range (i.e., they all use the entire estuary, Chabanne et al. 2017a), and the narrow geography of the Fremantle Inner Harbour and the adjoining lower reaches of the estuary make encounters likely (Chabanne et al. 2012). At a larger scale, the SCR dolphins are more related to each other than to those of adjacent communities (Chabanne et al. 2021), likely affecting analytical detectability of the influence of the homogeneously high relatedness on the strength of male bonds in SCR.
The strongly bonded males in this small community satisfied the criteria for being classified as first-order alliances, similar to those identified in other studies (e.g., Connor et al. 1992a, b; Ermak et al. 2017). Allies within each alliance (the triad ARR/BOT/HII and the dyad EXT/PRI) were ranked closest or second closest to each other, and their bonds were described as long-term preferred and stable associations. Two single males (PEB and KWL) shared moderately strong associations (SRI > 0.20; e.g., Ermak et al. 2017; King et al. 2018) with one first-order alliance (EXT/PRI). However, their lack of mutually strong bonds does not qualify them as members of a second-order alliance, which is defined by at least two first-order alliances having moderate associations (Connor et al. 1992a, b). In addition, KWL’s true affiliation (GAI) suggested that his association was primarily due to being highly gregarious (i.e., avoidance with all males when corrected for gregariousness) compared to the other males (Appendix 1). With only eight adult males residing in the SCR, the presence of only one alliance level is perhaps unsurprising. It is likely that there is a minimum encounter rate within a population at which the social structure might evolve to include more than one level of alliance formation (Whitehead and Connor 2005; Connor et al. 2017, 2019; Table A1). This is supported by the fact that formation of multi-level alliances in bottlenose dolphin populations has thus far only been reported from Shark Bay, Western Australia, and St. John’s River, Florida, sites which have some of the highest reported densities and/or conspecific encounter rates in the genus Tursiops (e.g., Nicholson et al. 2012; Ermak et al. 2017).
Three criteria are typically proposed as driving the formation of male alliances in dolphin populations: high density or encounter rate, male-biased operational sex ratio, and little or no sexual size dimorphism (Whitehead and Connor 2005; Möller 2012). There is no sexual dimorphism in the community (unpub. data). However, density appears to fall within the range of those populations that do not exhibit male alliance formation (Table A1, e.g., Brusa et al. 2016; Baker et al. 2019).
Nevertheless, several factors complicate our understanding of the drivers of alliance formation in the SCR. First, the community is not strictly estuarine, but coastal-estuarine, as males occasionally range to the nearest adjacent coastal areas throughout the year, thereby gaining access to receptive females (i.e., they are not limited to females from the estuary only). Second, despite some level of genetic structure between communities, the SCR community is genetically connected to adjacent coastal communities (Chabanne et al. 2021), suggesting that male alliance formation may be best understood in the social-environment context of the broader coastal population. Third, the SCR is a relatively narrow estuarine river system, which may drive encounter rates up, thus favouring male alliance formation (Connor and Whitehead 2005).
Several studies have affirmed that social factors are more important than environmental factors in the evolution of complex coalitions in mammals (Olson and Blumstein 2009; Ostner and Schülke 2014). However, He et al. (2019) highlighted a more complex evolutionary system in animal societies situated within environments where habitat configuration can drive social and ecological factors. Dolphins in the SCR appear to have stronger and more enduring associations with their peers than do dolphins in coastal habitats (Chabanne et al. 2017a). The shallow and protected estuary habitat provides resources that allow dolphins to reside year-round, with prey availability being continuous and more dependable than that in coastal habitats (McCluskey et al. 2016). Habitat and prey selection is therefore likely to influence how dolphins associate (Holyoake et al. 2010; O'Brien et al. 2020; McCluskey et al. 2021; Nicholson et al. 2021).
The difficulties in inferring the determinants of alliance formation through photo-identification-based studies are amplified by the small size of the SCR community. Studies such as this may obtain a rich, long-term, individual-specific dataset of social behaviour, which includes both group association data collected systemically and opportunistic behavioural observations. However, such studies might then be restricted in terms of the quantitative social analyses that can be conducted because of the small sample sizes and, thus, lack of power. Further, the small size of a community or population, and its discreteness as a socio-ecological unit, may–as in SCR–reflect the unique social and ecological milieu in which the community occurs (e.g., Giménez et al. 2018). Likewise, the uniqueness of this socio-ecological context and the small community/population size means that the factors determining male alliance formation may be exceptional in small populations, in the sense that the general relationships between social and ecological determinants of alliance formation might not apply. In the SCR, for example, the geography of the estuary (i.e., narrow area), the small community size, and occasional coastal-estuarine ranging patterns of males are factors that are distinctively different than those for the larger coastal communities nearby (Chabanne et al. 2017a).
Photo-identification of individually recognizable mammals is a versatile and powerful approach for the field study of marine and terrestrial mammals and has provided significant insights into our understanding of mammalian ecology and behaviour. Although our results were limited by the small size of the community in the SCR, the apparent occurrence of male alliances across the coastal waters of the Perth region provides a promising opportunity to extend our understanding of the evolutionary and ecological processes driving alliance formation. A larger, comparative approach, in which genetic, environmental, and behavioural data from several populations are used to model parameters that are predictive of complex alliances will help us to understand the evolutionary drivers of alliance formation. The SCR dolphins could serve as one important piece in this puzzle.
Data availability
Given the long-term and ongoing research on the dolphin community in the Swan Canning Riverpark, data will be made available upon request to the corresponding author (DBHC).
Code availability
R scripts for analysis are available in Figshare.
References
Allen SJ, Cagnazzi DD, Hodgson AJ, Loneragan NR, Bejder L (2012) Tropical inshore dolphins of north-western Australia: unknown populations in a rapidly changing region. Pac Conserv Biol 18:56–63. https://doi.org/10.1071/PC120056
Allen SJ, Bryant KA, Kraus RH, Loneragan NR, Kopps AM, Brown AM, Gerber L, Krützen M (2016) Genetic isolation between coastal and fishery-impacted, offshore bottlenose dolphin (Tursiops spp.) populations. Mol Ecol 25:2735–2753. https://doi.org/10.1111/mec.13622
Allen SJ, King SL, Krutzen M, Brown AM (2017) Multi-modal sexual displays in Australian humpback dolphins. Sci Rep 7:13644. https://doi.org/10.1038/s41598-017-13898-9
Baker I, O’Brien J, McHugh K, Berrow S (2017) Female reproductive parameters and population demographics of bottlenose dolphins (Tursiops truncatus) in the Shannon Estuary, Ireland. Mar Biol 165:15. https://doi.org/10.1007/s00227-017-3265-z
Baker I, O’Brien J, McHugh K, Berrow S (2019) Fine-scale sociality reveals female–male affiliations and absence of male alliances in bottlenose dolphins (Tursiops truncatus) in the Shannon Estuary, Ireland. Mar Mammal Sci 36:66–88. https://doi.org/10.1111/mms.12631
Bejder L, Fletchert D, Brager S (1998) A method for testing association patterns of social animals. Anim Behav 56:719–725. https://doi.org/10.1006/anbe.1998.0802
Bizzozzero MR, Allen SJ, Gerber L, Wild S, King SL, Connor RC, Friedman WR, Wittwer S, Krutzen M (2019) Tool use and social homophily among male bottlenose dolphins. Proc Royal Soc B 286:20190898. https://doi.org/10.1098/rspb.2019.0898
Bouveroux T, Mallefet J (2010) Social structure of bottlenose dolphins, Tursiops truncatus, in Panama City, Florida. J Mar Biol Assoc U K 90:1685–1692. https://doi.org/10.1017/S0025315409991251
Bridge PD (1993) Classification. In: Fry JC (ed) Biological data analysis. Oxford University Press, Oxford, UK, pp 219–242
Brown AC, Bejder L, Cagnazzi D, Parra GJ, Allen SJ (2012) The North West Cape, Western Australia: a potential hotspot for Indo-Pacific humpback dolphins Sousa chinensis? Pac Conserv Biol 18:240–246. https://doi.org/10.1071/PC120240
Brown AM, Bejder L, Parra GJ, Cagnazzi D, Hunt T, Smith JL, Allen SJ (2016a) Sexual dimorphism and geographic variation in dorsal fin features of Australian humpback dolphins, Sousa sahulensis. Adv Mar Biol 73:273–314. https://doi.org/10.1016/bs.amb.2015.08.002
Brown AM, Bejder L, Pollock KH, Allen SJ (2016b) Site-specific assessments of the abundance of three inshore dolphin species to inform conservation and management. Front Mar Sci 3:4. https://doi.org/10.3389/fmars.2016.00004
Brusa JL, Young RF, Swanson T (2016) Abundance, ranging patterns, and social behavior of bottlenose dolphins (Tursiops truncatus) in an estuarine terminus. Aquat Mamm 42:109–121. https://doi.org/10.1578/AM.42.1.2016.109
Cairns SJ, Schwager SJ (1987) A comparison of assocation indices. Anim Behav 35:1454–1469. https://doi.org/10.1016/S0003-3472(87)80018-0
Calenge C (2006) The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecol Modell 197:516–519. https://doi.org/10.1016/j.ecolmodel.2006.03.017
Chabanne D, Finn H, Salgado-Kent C, Bejder L (2012) Identification of a resident community of bottlenose dolphins (Tursiops aduncus) in the Swan Canning Riverpark, Western Australia, using behavioural information. Pac Conserv Biol 18:247–262. https://doi.org/10.1071/PC120247
Chabanne DBH, Finn H, Bejder L (2017a) Identifying the relevant local population for environmental impact assessments of mobile marine fauna. Front Mar Sci 4:148. https://doi.org/10.3389/fmars.2017.00148
Chabanne DBH, Pollock KH, Finn H, Bejder L (2017b) Applying the multistate capture-recapture robust design to characterize metapopulation structure. Methods Ecol Evol 8:1547–1557. https://doi.org/10.1111/2041-210X.12792
Chabanne DBH, Allen SJ, Sherwin WB, Finn H, Krützen M (2021) Inconsistency between socio-spatial and genetic structure in a coastal dolphin population. Front Mar Sci 7:1217. https://doi.org/10.3389/fmars.2020.617540
Connor RC, Krützen M (2015) Male dolphin alliances in Shark Bay: changing perspectives in a 30-year study. Anim Behav 103:223–235. https://doi.org/10.1016/j.anbehav.2015.02.019
Connor R, Whitehead H (2005) Alliances II rates of encounter during resource utilization: a general model of intrasexual alliance formation in fission–fusion societies. Anim Behav 69:127–132. https://doi.org/10.1016/j.anbehav.2004.02.022
Connor RC, Smolker RA, Richards AF (1992a) Dolphin alliances and coalitions. In: Harcourt A, de Waal F (eds) Coalitions in humans and other animals. Oxford Univesrity Press, New York, pp 415–443
Connor RC, Smolker RA, Richards AF (1992b) Two levels of alliance formation among male bottlenose dolphins (Tursiops sp.). Proc Natl Acad Sci USA 89:987–990. https://doi.org/10.1073/pnas.89.3.987
Connor RC, Heithaus MR, Barre LM, Spoor F, Higgins PO, Dean C, Lieberman DE (1999) Superalliance of bottlenose dolphins. Nature 397:571–572. https://doi.org/10.1038/17501
Connor RC, Read AJ, Wrangham R (2000) Male reproductive strategies and social bonds. In: Mann J, Connor RC, Tyack PL, Whitehead H (eds) Cetacean societies: field studies of dolphins and whales. The University of Chicago Press, Chicago, IL, pp 247–269
Connor RC, Watson-Capps JJ, Sherwin WB, Krützen M (2011) A new level of complexity in the male alliance networks of Indian Ocean bottlenose dolphins (Tursiops sp.). Biol Lett 7:623–626. https://doi.org/10.1098/rsbl.2010.0852
Connor RC, Cioffi WR, Randic S, Allen SJ, Watson-Capps J, Krützen M (2017) Male alliance behaviour and mating access varies with habitat in a dolphin social network. Sci Rep 7:46354. https://doi.org/10.1038/srep46354
Connor RC, Sakai M, Morisaka T, Allen SJ (2019) The Indo-Pacific Bottlenose Dolphin (Tursiops aduncus). In: Würsig B (ed) Ethology and behavioral ecology of odontocetes. Springer Nature, Switzerland AG, pp 345–368
Connor RC, Richards AF, Smolker RA, Mann J. (1996) Patterns of female attractiveness in Indian ocean bottlenose dolphins. Behaviour 133: 37–69. https://www.jstor.org/stable/4535343
Diaz-Aguirre F, Parra GJ, Passadore C, Möller L (2018) Kinship influences social bonds among male southern Australian bottlenose dolphins (Tursiops cf. australis). Behav Ecol Sociobiol 72:190. https://doi.org/10.1007/s00265-018-2621-4
Elliser CR, Herzing DL (2011) Replacement dolphins? Social restructuring of a resident pod of Atlantic bottlenose dolphins, Tursiops truncatus, after two major hurricanes. Mar Mammal Sci 27:39–59. https://doi.org/10.1111/j.1748-7692.2010.00403.x
Ermak J, Brightwell K, Gibson Q (2017) Multi-level dolphin alliances in northeastern Florida offer comparative insight into pressures shaping alliance formation. J Mammal 98:1096–1104. https://doi.org/10.1093/jmammal/gyx053
Fieberg J, Kochanny C (2005) Quantifying home-range overlap the importance of the utilization distribution. J Wildl Manage 69: 1346–1359. https://www.jstor.org/stable/3803498
Forcada J, Aguilar A (2000) Use of photographic identification in capture-recapture studies of Mediterranean monk seals. Mar Mammal Sci 16:767–793. https://doi.org/10.1111/j.1748-7692.2000.tb00971.x
Genov T, Centrih T, Kotnjek P, Hace A (2019) Behavioural and temporal partitioning of dolphin social groups in the northern Adriatic Sea. Mar Biol 166:11. https://doi.org/10.1007/s00227-018-3450-8
Genov T, Kotnjek P, Lesjak J, Hace A, Fortuna CM. (2008) Bottlenose dolphins (Tursiops truncatus) in Slovenian and adjacent waters (Northern Adriatic Sea). Annales 227–244
Gerber L, Connor RC, King SL, Allen SJ, Wittwer S, Bizzozzero MR, Friedman WR, Kalberer S, Sherwin WB, Wild S, Willems EP, Krutzen M (2020) Affiliation history and age similarity predict alliance formation in adult male bottlenose dolphins. Behav Ecol 31:361–370. https://doi.org/10.1093/beheco/arz195
Giménez J, Louis M, Barón E, Ramírez F, Verborgh P, Gauffier P, Esteban R, Eljarrat E, Barceló D, Manuela GF, de Stephanis R (2018) Towards the identification of ecological management units: a multidisciplinary approach for the effective management of bottlenose dolphins in the southern Iberian Peninsula. Aquat Conserv Mar Freshw Ecosyst 28:205–215. https://doi.org/10.1002/aqc.2814
Ginsberg MP, Young TP (1992) Measuring association between individuals or groups in behavioural studies. Anim Behav 44:377–379. https://doi.org/10.1016/0003-3472(92)90042-8
Godde S, Humbert L, Côté SD, Réale D, Whitehead H (2013) Correcting for the impact of gregariousness in social network analyses. Anim Behav 85:553–558. https://doi.org/10.1016/j.anbehav.2012.12.010
Hammond PS, Mizroch SA, Donovan GP. (1990) Individual recognition of cetaceans: use of photo-identification and other techniques to estimate population parameters. Report of the International Whaling Commission pp 440
Hartman KL, Visser F, Hendriks AJE (2008) Social structure of Risso’s dolphins (Grampus griseus) at the Azores: a stratified community based on highly associated social units. Can J Zool 86:294–306. https://doi.org/10.1139/z07-138
Haughey R, Hunt T, Hanf D, Rankin RW, Parra GJ (2020) Photographic capture-recapture analysis reveals a large population of Indo-Pacific bottlenose dolphins (Tursiops aduncus) with low site fidelity off the North West Cape, Western Australia. Front Mar Sci 6:781. https://doi.org/10.3389/fmars.2019.00781
He P, Maldonado-Chaparro AA, Farine DR (2019) The role of habitat configuration in shaping social structure: a gap in studies of animal social complexity. Behav Ecol Sociobiol 73:9. https://doi.org/10.1007/s00265-018-2602-7
Henderson SD, Dawson SM, Currey RJC, Lusseau D, Schneider K (2014) Reproduction, birth seasonality, and calf survival of bottlenose dolphins in Doubtful Sound, New Zealand. Mar Mammal Sci 30:1067–1080. https://doi.org/10.1111/mms.12109
Holyoake C, Finn H, Stephens N, Duignan P, Salgado C, Smith H, Bejder L, Linke T, Daniel C, Lo HN, Ham GS, Moiler K, Allen S, Bryant K, McElligott D (2010) Technical report on the bottlenose dolphin (Tursiops aduncus) unusual mortality event within the Swan Canning Riverpark. June-October 2009:234
Hunt TN, Allen SJ, Bejder L, Parra GJ (2019) Assortative interactions revealed in a fission–fusion society of Australian humpback dolphins. Behav Ecol 30:914–927. https://doi.org/10.1093/beheco/arz029
Killick R, Eckley IA (2014) Changepoint: an R package for changepoint analysis. J Stat Soft 58:1–19. https://doi.org/10.18637/jss.v058.i03
King SL, Friedman WR, Allen SJ, Gerber L, Jensen FH, Wittwer S, Connor RC, Krutzen M (2018) Bottlenose Dolphins retain individual vocal labels in multi-level alliances. Curr Biol. https://doi.org/10.1016/j.cub.2018.05.013
King SL, Connor RC, Krützen M, Allen SJ (2021) Cooperation-based concept formation in male bottlenose dolphins. Nat Commun 12:2373. https://doi.org/10.1038/s41467-021-22668-1
Kogi K, Hishii T, Imamura A, Iwatani T, Dudzinski KM (2004) Demographic parameters of Indo-Pacific bottlenose dolphins (Tursiops aduncus) around Mikura Island. Japan 20:510–526. https://doi.org/10.1111/j.1748-7692.2004.tb01176.x
Krützen M, Barre LM, Moller LM, Heithaus MR, Simms C, Sherwin WB (2002) A biopsy system for small cetaceans: darting success and wound healing in Tursiops spp. Mar Mammal Sci 18:863–878. https://doi.org/10.1111/j.1748-7692.2002.tb01078.x
Krutzen M, Barre LM, Connor RC, Mann J, Sherwin WB (2004) 'O father: where art thou?’–Paternity assessment in an open fission-fusion society of wild bottlenose dolphins (Tursiops sp.) in Shark Bay, Western Australia. Mol Ecol 13:1975–1990. https://doi.org/10.1111/j.1365-294X.2004.02192.x
Le Pendu Y, Ciofolo I, Gosser A (2000) The social organization of giraffes in Niger. Afr J Ecol 38:78–85. https://doi.org/10.1046/j.1365-2028.2000.00214.x
Lebreton J-D, Burnham KP, Clobert J, Anderson DR (1992) Modeling survival and testing biological hypotheses using marked animals a unified approach with case studies. Ecol Monogr 62:67–118. https://doi.org/10.2307/2937171
Lusseau D (2007) Why are male social relationships complex in the doubtful sound bottlenose dolphin population? PLoS One 2:e348. https://doi.org/10.1371/journal.pone.0000348
Mann J (1999) Behavioral sampling methods for cetaceans: a review and critique. Mar Mammal Sci 15:102–122. https://doi.org/10.1111/j.1748-7692.1999.tb00784.x
McCluskey SM, Bejder L, Loneragan NR (2016) Dolphin prey availability and calorific value in an estuarine and coastal environment. Front Mar Sci 3:30. https://doi.org/10.3389/fmars.2016.00030
McCluskey SM, Sprogis KR, London JM, Bejder L, Loneragan NR (2021) Foraging preferences of an apex marine predator revealed through stomach content and stable isotope analyses. Glob Ecol Conserv 25:e01396. https://doi.org/10.1016/j.gecco.2020.e01396
Meekan MG, Bradshaw CJA, Press M, McLean C, Richards A, Quasnichka S, Taylor JG (2006) Population size and structure of whale sharks Rhincodon typus at Ningaloo Reef, Western Australia. Mar Ecol Prog Ser 319:275–285. https://doi.org/10.3354/meps319275
Meyer D, Zeileis A, Hornik K. (2021) vcd: visualizing categorical data. R package version 1.4–9
Möller LM (2001) Social organisation and genetic relationships of coastal botlenose dolphins in southeastern Australia, vol 392. PhD Thesis Macquarie University, Sydney, Australia, p 392
Möller LM (2012) Sociogenetic structure, kin associations and bonding in delphinids. Mol Ecol 21:745–764. https://doi.org/10.1111/j.1365-294X.2011.05405.x
Möller LM, Allen SJ, Harcourt RG (2002) Group characteristics, site fidelity and seasonal abundance of bottlenose dolphins Tursiops aduncus in Jervis Bay and Port Stephens, south-eastern Australia. Aust Mammal 24:11–21. https://doi.org/10.1071/AM02011
Nicholson K, Bejder L, Allen SJ, Krützen M, Pollock KH (2012) Abundance, survival and temporary emigration of bottlenose dolphins (Tursiops sp.) off Useless Loop in the western gulf of Shark Bay, Western Australia. Mar Freshw Res 63:1059–1068. https://doi.org/10.1071/mf12210
Nicholson K, Bejder L, Loneragan N (2021) Niche partitioning among social clusters of a resident estuarine apex predator. Behav Ecol Sociobiol 75:160. https://doi.org/10.1007/s00265-021-03091-4
Nishita M, Shirakihara M, Iwasa N, Amano M (2017) Alliance formation of Indo-Pacific bottlenose dolphins (Tursiops aduncus) off Amakusa, Western Kyushu, Japan. Mammal Study 42:125–130. https://doi.org/10.3106/041.042.0302
O’Brien O, Allen SJ, Krützen M, Connor RC (2020) Alliance-specific habitat selection by male Indo-Pacific bottlenose dolphins in Shark Bay, Western Australia. Anim Behav 164:39–49. https://doi.org/10.1016/j.anbehav.2020.03.014
Olson LE, Blumstein DT (2009) A trait-based approach to understand the evolution of complex coalitions in male mammals. Behav Ecol 20:624–632. https://doi.org/10.1093/beheco/arp040
Ostner J, Schülke O (2014) The evolution of social bonds in primate males. Behaviour 151:871–906. https://doi.org/10.1163/1568539x-00003191
Owen ECG, Wells RS, Hofmann S (2002) Ranging and association patterns of paired and unpaired adult male Atlantic bottlenose dolphins, Tursiops truncatus, in Sarasota, Florida, provide no evidence for alternative male strategies. Can J Zool 80:2072–2089. https://doi.org/10.1139/z02-195
Parsons KM, Durban JW, Claridge DE, Balcomb KC, Noble LR, Thompson PM (2003) Kinship as a basis for alliance formation between male bottlenose dolphins, Tursiops truncatus, in the Bahamas. Anim Behav 66:185–194. https://doi.org/10.1006/anbe.2003.2186
Passadore C, Möller L, Diaz-Aguirre F, Parra GJ (2017) Demography of southern Australian bottlenose dolphins living in a protected inverse estuary. Aquat Conserv: Mar Freshw Ecosyst 27:1186–1197. https://doi.org/10.1002/aqc.2772
Petersen JCB (1972) An identification system for zebra (Equus burchelli, Gray). Afr J Ecol 10:59–63. https://doi.org/10.1111/j.1365-2028.1972.tb00858.x
Pew J, Muir PH, Wang J, Frasier TR (2015) related: an R package for analysing pairwise relatedness from codominant molecular markers. Mol Ecol Resour 15:557–561. https://doi.org/10.1111/1755-0998.12323
Quintana-Rizzo E, Wells RS (2001) Resighting and association patterns of bottlenose dolphins (Tursiops truncatus) in the Cedar Keys, Florida: insights into social organization. Can J Zool 79:447–456. https://doi.org/10.1139/z00-223
Randić S, Connor RC, Sherwin WB, Krützen M, Randic S (2012) A novel mammalian social structure in Indo-Pacific bottlenose dolphins (Tursiops sp.): complex male alliances in an open social network. Proc Royal Soc B 279:3083–3090. https://doi.org/10.1098/rspb.2012.0264
Raudino HC, Douglas CR, Waples KA (2018) How many dolphins live near a coastal development? Reg Stud Mar Sci 19:25–32. https://doi.org/10.1016/j.rsma.2018.03.004
Read AJ, Wells RS, Hohn AA, Scott MD (1993) Patterns of growth in wild bottlenose dolphins, Tursiops truncatus. J Zool 231:107–123. https://doi.org/10.1111/j.1469-7998.1993.tb05356.x
Scott EM, Mann J, Watson-Capps JJ, Sargeant BL, Connor RC. (2005) Aggression in bottlenose dolphins: evidence for sexual coercion, male-male competition, and female tolerance through analysis of tooth-marks and behaviour. Behaviour 142: 21–44. https://www.jstor.org/stable/4536227
Smolker RA, Richards AF, Connor RC, Pepper JW. (1992) Sex differences in patterns of association among Indian ocean bottlenose dolphins. 123: 38–69. https://www.jstor.org/stable/4535060
Sprogis KR, Raudino HC, Rankin R, MacLeod CD, Bejder L (2015) Home range size of adult Indo-Pacific bottlenose dolphins (Tursiops aduncus) in a coastal and estuarine system is habitat and sex-specific. Mar Mammal Sci 32:287–308. https://doi.org/10.1111/mms.12260
Team RC. (2013) R: a language and environment for statistical computing
Tsai Y-JJ, Mann J (2013) Dispersal, philopatry, and the role of fission-fusion dynamics in bottlenose dolphins. Mar Mammal Sci 29:261–279. https://doi.org/10.1111/j.1748-7692.2011.00559.x
Wells RS, Scott MD, Irvine AB, Genoways H (1987) The social structure of free-ranging bottlenose dolphins. In: Genoways HH (ed) Current mammalogy. Springer, Boston, MA, pp 247–305
Whitehead H (1995) Investigating structure and temporal scale in social organizations using identified individuals. Behav Ecol 6:199–208. https://doi.org/10.1093/beheco/6.2.199
Whitehead H (2008a) Analyzing animal societies: quantitative methods for vertebrate social analysis. Chicago University Press, Chicago, IL
Whitehead H (2008b) Precision and power in the analysis of social structure using associations. Anim Behav 75:1093–1099. https://doi.org/10.1016/j.anbehav.2007.08.022
Whitehead H, Connor R (2005) Alliances I. How large should alliances be? Anim Behav 69:117–126. https://doi.org/10.1016/j.anbehav.2004.02.021
Whitehead H, Dufault S (1999) Techniques for analyzing vertebrate social structure using identified individuals: review and recommendations. Adv Study Behav 28:33–74. https://doi.org/10.1016/s0065-3454(08)60215-6
Whitehead H, James R (2015) Generalized affiliation indices extract affiliations from social network data. Methods Ecol Evol 6:836–844. https://doi.org/10.1111/2041-210X.12383
Whitehead H, Bejder L, Andrea Ottensmeyer C (2005) Testing association patterns: issues arising and extensions. Anim Behav. https://doi.org/10.1016/j.anbehav.2004.11.004
Whitehead H. (2019) SOCPROG 2.9: programs for analyzing social structure
Wiszniewski J, Brown C, Möller LM (2012a) Complex patterns of male alliance formation in a dolphin social network. J Mammal 93:239–250. https://doi.org/10.1644/10-mamm-a-366.1
Wiszniewski J, Corrigan S, Beheregaray LB, Moller LM (2012b) Male reproductive success increases with alliance size in Indo-Pacific bottlenose dolphins (Tursiops aduncus). J Anim Ecol 81:423–431. https://doi.org/10.1111/j.1365-2656.2011.01910.x
Würsig B, Jefferson TA (1990) Methods of photo-identification for small cetaceans. In: Hammond PS, Mizroch SA, Donovan GP (eds) Individual recognition of cetaceans: use of photo-identification and other techniques to estimate population parameters, vol 12. Reports of the International Whaling Commission Special Issue, Cambridge, pp 43–52
Acknowledgements
The authors would like to dedicate this manuscript to the late Cathie O’Neill, who financially supported this study and founded the Swan Estuary Reserves Action Group. Cathie was an incredible advocate for protecting the environment around the Swan Canning Riverpark and will be sorely missed. We further acknowledge the Whadjuk People of the Noongar Nation as the traditional custodians of the lands and waters on which this research took place, including the Derbal Nara, Derbal Yerrigan, and Djarlgarra. We thank Lars Bejder for his contribution during DBHC’s Ph.D. We also thank many field assistants, without whom this research would not have been possible. We would also like to thank the Fremantle Sailing Club for logistical support. Field research was conducted under the conditions of licenses, authorities, and permits from the Western Australia Department of Biodiversity, Conservation and Attractions (SF005997, SF006538, SF007046, SF007596, SF008480, SF009119, SF009734, and SF0101223) and the Murdoch University Animal Ethics Committee (W2076/07, W2307/10, W2342/10, and R2649/14). Finally, we thank the editors and reviewers for their constructive and helpful comments, which improved this manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Funding for this research was provided by the Swan River Trust and the Department of Biodiversity, Conservation and Attractions (grant numbers IRMA: 15544, 16207, 17707 and 19031) with additional support from Fremantle Ports and Catherine O’Neill. DBHC also acknowledges the Australian Postgraduate Award from Murdoch University (Ph.D.) and the Swiss Government Excellence Scholarship (Postdoctoral Fellowship).
Author information
Authors and Affiliations
Contributions
DBHC conceived and designed the study, collected, and processed the data. DBHC analysed the data with advice from MK and SJA. DBHC wrote the manuscript, with contributions to drafting, review and editorial input from MK, HF and SJA. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
The animal study was reviewed and approved by the Animal Ethics Committee, Murdoch University.
Consent to participate
N/A.
Consent for publication
N/A.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling editors: Stephen C.Y. Chan and Scott Y.S. Chui.
This article is a contribution to the special issue on “Individual Identification and Photographic Techniques in Mammalian Ecological and Behavioural Research – Part 2: Field Studies and Applications” — Editors: Leszek Karczmarski, Stephen C.Y. Chan, Scott Y.S. Chui and Elissa Z. Cameron.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix 1
See Table A1.
Appendix 2
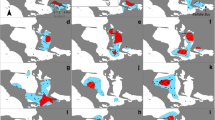
Maps representing the 95% kernel density estimated for each of the eight males residing in the SCR and using sighting data from boat-based surveys conducted in the SCR from 2011 to 2017. In bold are the three-letter codes identifying each male (see Supplementary Material no. 1 for individual male history). The predetermined boat-based transect line is shown on the first map (top left)
See Fig. A1.
Appendix 3: Evaluation of the true associations between male dolphins residing in the Swan Canning Riverpark using the Generalized Association Indices (GAI)
Based on the MRQAP test, values of SRIs were significantly correlated to gregariousness while controlling for home range overlap and relatedness. We therefore verified the strength and true preferred associations among males using the deviance residual of the generalized affiliation indices (GAIs). GAIs are the residuals of a generalized linear model that was built with SRI as the dependent variable and gregariousness as the structural variable (Whitehead and James 2015) and allow the assessment of the true social affiliations unaffected by the gregariousness that could confound why some males have strong associations (Whitehead and James 2015).
As with SRI values, we tested for the existence of long-term preferences between males by following the Monte-Carlo resampling (i.e., permutation test) procedure established by Whitehead et al. (2005). With GAIs, we considered the pattern significant when the standard deviation of the observed associations was higher than expected by chance (Whitehead and James 2015). Pairs of males with a positive value of the deviance residual of GAI were considered preferred companionships given the structural predictor variables while those with negative values indicated avoidance.
The mean and maximum affiliation indices (GAIs) were 0.01 (SD 0.11) and 0.21 (SD 0.23), respectively. The permutation test for long-term preferred associations among males was significant (SD real data = 0.185; SD random data = 0.147, p value < 0.0001). Among the closest associates, the triad ARR/BOT/HII had high positive deviance residuals (ranging from 5.90 to 6.61) supporting their strong affiliations. The dyad EXT/PRI followed with a positive deviance residual of 1.92. PEB has positive residual deviances with each member of the dyad, although the values (lower than 1.50) would best describe for casual companionships (e.g., Hunt et al. 2019). Since the difference between the residual deviances of the male BLA with the triad were large (minimum difference of 4.06 compared to a maximum difference of 0.71 between the triad), he would best be considered as a casual companion to the triad. The residual deviances values of KWL with the dyad (and any other males) revealed a strong effect of his gregariousness (GAI < − 1.50), with KWL significantly avoiding all males (Fig. A2).
Dendrogram produced using hierarchical average cluster analysis with GAIs (CCC = 0.96682) of the eight male bottlenose dolphins (i.e., three-letter code) in the SCR. Levels of affiliation are displayed above with the dashed black vertical lines indicating the thresholds (− 1.50 and 1.50). Coloured solid lines denote the males forming strong affiliation (GAI > 1.50 describing for a dyad and a triad). Black solid lines are non-significant relationships while black round dot lines denote avoidance
Appendix 4
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chabanne, D.B.H., Krützen, M., Finn, H. et al. Evidence of male alliance formation in a small dolphin community. Mamm Biol 102, 1285–1298 (2022). https://doi.org/10.1007/s42991-022-00295-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-022-00295-7