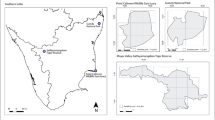
Abstract
Reliable estimates of population size and a knowledge of determinants of detectability and density estimates are crucial for effective conservation of species. Using Multiple Covariate Distance Sampling (MCDS), we modelled the influence of covariates on detection probability and density estimates of native blackbuck Antilope cervicapra and the invasive feral-horse Equus caballus at Point Calimere Wildlife Sanctuary. Grids of 1 × 1 km size were overlaid on the study area with a 1-km line transect in alternate grid cells. Sixteen transects were walked four times each, which detected 199 blackbuck and 152 feral-horse clusters. On each sighting, the climatic, habitat and anthropogenic covariates were recorded, which are likely to affect detection probability. At first, exploratory analyses were made using Conventional Distance Sampling (CDS) to arrive at estimates. Later, in Multiple Covariate Distance Sampling (MCDS), key models were fit into the dataset after selecting the potential covariates that had a significant effect on detection distances obtained from FAMD/PCA, and the best one was selected based on AIC. The MCDS analysis in blackbuck included covariates, viz., distance to water in the best model (29 individuals/km2; CI 22–36; detection probability = 0.58), followed by distance to feral-horse, sampling time and principal diet. The covariate distance to water emerged as the best model for feral-horse as well (13 individuals/km2; CI 9–18; detection probability = 0.36), followed by distance to cattle and principal diet. While the MCDS approach outperformed the CDS global and survey-strata estimates in blackbuck, both the approaches had a marginal difference in feral-horse. Post-stratification analysis showed that blackbuck density increased significantly with distance to water and feral-horse, but insignificantly with the absence of principal diet; whereas, the density of the feral-horse increased significantly with distance to water and cattle in the presence of principal diet. These findings suggest the ability of feral-horse to keep the blackbuck away from optimal areas, where the principal diet is abundant. Our study, thus, illustrates the need for the use of MCDS approach that ascertained (i) reliable population density estimates, (ii) spatiotemporal constraint on large herbivores caused by overabundance of water during the wet season, and (iii) competitive interaction of the invasive feral-horse with the native blackbuck and its likely effect on the latter keeping away from the former and the principal diet to overcome competition. Thus, the study highlights the impact of feral-horse on the native species and suggests measures for the long-term conservation of the blackbuck.





Similar content being viewed by others
References
Ali R (2005) Field studies for the conservation and management of Point Calimere Complex. Foundation for Ecological Research, Advocacy and Learning. A Report for the Tamil Nadu Forest Department, p 44
Anderson DR, Burnham KP (2002) Avoiding pitfalls when using information-theoretic methods. J Wildl Manag 66:912–918
Anderson DR, Burnham KP, Lubow BC, Thomas L, Corn PS, Medica PA, Marlow RW (2001) Field trials of line transect methods applied to estimation of desert tortoise abundance. J Wildl Manag 65:583–597
Annual Census (2018) Annual Census for Blackbuck (Antilope cevicapra) and Spotted Deer (Axis axis) in Guindy National Park, Chennai, Tamil Nadu, February, 2018. In Divisional Forest Office Guindy, Tamil Nadu Forest Department, p 16
Annual Census (2019) Annual Census for Blackbuck (Antilope cevicapra) and Spotted Deer (Axis axis) in Guindy National Park, Chennai, Tamil Nadu, February, 2019. In Divisional Forest Office Guindy, Tamil Nadu Forest Department, p 16
Atwood TC, Gese EM (2010) Importance of resource selection and social behavior to partitioning of hostile space by sympatric canids. J Mammal 91(2):490–499
Banks MA (2013) Determinants of abundance and the distribution of primates in northern Madagascar (Physical Anthropology). Stony Brook University, Stony Brook
Baskaran N, Anbarasan U, Agoramoorthy G (2012) India's biodiversity hotspot under anthropogenic pressure: a case study of Nilgiri Biosphere Reserve. J Nat Conserv 20(1):56–61
Baskaran N, Ramkumaran K, Karthikeyan G (2016) Spatial and dietary overlap between blackbuck (Antilope cervicapra) and feral horse (Equus caballus) at Point Calimere Wildlife Sanctuary, Southern India: Competition between native versus introduced species. Mamm Biol 81(3):295–302
Baskaran N, Arandhara S, Sathishkumar S (2018) Technical report submitted to Department of Science and Technology, SERB, India. April 2018, Unpublished, p 7
Baskaran N, Arandhara, Sathishkumar S (2020) Monitoring report submitted to Department of Science and Technology, SERB, India. Jan, 2020, Unpublished, p 10
Bell RHV (1969) The use of the herbaceous layer by grazing ungulates in the Serengeti National Park, Tanzania. PhD thesis, University of Manchester
Bell RHV (1971) A grazing ecosystem in the Serengeti. Sci Am 225:86–93
Berger KM, Gese EM (2007) Does interference competition with wolves limit the distribution and abundance of coyotes? J Animal Ecol 76(6):1075–1085
Borchers DL, Buckland ST, Zucchini W (2002) Estimating animal abundance. Closed populations. Springer, London
Boulinier T, Nichols J, Sauer J, Hines J, Pollock K (1998) Estimating species richness: the importance of heterogeneity in species detectability. Ecology 79(3):1018–1028. https://doi.org/10.2307/176597
Buckland JF, Anderson DR, Burnham KP, Laake TL, Borchers DL, Thomas L (2001) Introduction to distance sampling: estimating abundance of biological populations. Oxford University Press, Oxford
Buckland ST, Anderson DR, Burnham KP, Laake JL (2005) Distance sampling. Encyclopaedia of biostatistics. Wiley, New York
Buckland ST, Borchers DL, Johnston A, Henrys PA, Marques TA (2007) Line transect methods for plant surveys. Biom 63(4):989–998
Buckland ST, Marsden S, Green RE (2008) Estimating bird abundance: making methods work. Bird Con Int 18:S91–S108. https://doi.org/10.1017/S0959270908000294
Buckland ST, Rexstad EA, Marques TA, Oedekoven CS (2015) Distance sampling: methods and applications. Springer, New York, p 277
Burnham K, Anderson D (2002) Model selection and multi-model Inference, 2nd edn. New York, Springer
Burnham KP, Anderson DR, Laake JL (1980) Estimation of density from line transect sampling of biological populations. Wildl Monogr 72:3–202
Case TJ, Gilpin ME (1974) Interference competition and niche theory. Proc Natl Acad Sci 71(8):3073–3077
Chundawat RS, Sharma K (2008) Tiger prey in a tropical dry forest: an assessment of abundance and of biomass estimation derived from distance sampling. J Bombay Nat Hist Soc 105(1):64–72
Cooke RS, Woodfine T, Petretto M, Ezard TH (2016) Resource partitioning between ungulate populations in arid environments. Ecol Evol 6(17):6354–6365
Crane KK, Smith MA, Reynolds D (1997) Habitat selection patterns of feral horses in southcentral Wyoming. J Range Manag 50:374–380
Cromsigt JP, Olff H (2006) Resource partitioning among savanna grazers mediated by local heterogeneity: an experimental approach. Ecology 87(6):1532–1541
Crosbie SP, Souza LE, Ernest HB (2011) Estimating western scrub-jay density in California by multiple-covariate distance sampling. The Condor 113(4):843–852
Daniels RR, Arivazhagan C (2008) The Indian blackbuck recovers from the brink of extinction in Chennai, India Indian blackbuckAntilope cervicaprais an antelope endemic to the Indian subcontinent, with an estimated 35,000 in the wild. The Wildlife (Protection) Act, 1972, of India places it in
Danvir RE (2000) Environmental and density-dependent effects on a northern Utah pronghorn population. In: Proceedings of the pronghorn antelope workshop 17, 36–41. Man., pp 374–380.
Duncan P (1992) Horses and grasses: the nutritional ecology of equids and their impact on the camargue. Springer, New York, p 287
Dutoit JT (1990) Feeding-height stratification among African browsing ruminants. Afric J Ecol 28(1):55–61
ESRI (2014) ArcMap 10.2. Redlands
Focardi S, Montanaro P, Isotti R, Ronchi F, Scacco M, Calmanti R (2005) Distance sampling effectively monitored a declining population of Italian roe deer Capreolus capreolus italicus. Oryx 39(4):421–428
Gandiwa E (2014) Vegetation factors influencing density and distribution of wild large herbivores in a southern African savannah. Afr J Ecol 52(3):274–283
Gangadharan A (2005) Density estimation and time trend analysis of large herbivores in Nagarhole, India, Doctoral dissertation, The University of St Andrews
Gause GF (1934) Experimental analysis of Vito Volterra’s mathematical theory of the struggle for existence. Science 79(2036):16–17
Gooch AM, Petersen SL, Collins GH, Smith TS, McMillan BR, Eggett DL (2017) The impact of feral horses on pronghorn behavior at water sources. J Arid Environ 138:38–43
Gordon IJ, Illius AW (1989) Resource partitioning by ungulates on the Isle of Rhum. Oecol 79(3):383–389
Hall LK, Larsen RT, Westover MD, Day CC, Knight RN, McMillan BR (2016) Influence of exotic horses on the use of water by communities of native wildlife in a semi-arid environment. J Arid Environ 127:100–105
Hafez ESE, Williams M, Wierzbowski S (1969) The behavior of horses. In: Hafez ESE (ed) The behavior of domestic animals. Balliere, Tindall, and Cassell, London, pp 391–416
Hailey TL, Thomas JW, Robinson RM (1966) Pronghorn die-off in trans-Pecos Texas. J Wildl Manag 30:488–496
Henke SE, Demarais S, Pfister JA (1988) Digestive capacity and diets of white-tailed deer and exotic ruminants. J Wildl Manag 52:595–598
Hofmann RR (1989) Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view of their digestive system. Oecology 78(4):443–457
Hofmann RR, Stewart DRM (1972) Grazer or browser: a classification based on the stomach structure and feeding habits of East African ruminants. Mammalia 36:226–240
Holt RD, Polis GA (1997) A theoretical framework for intraguild predation. Am Nat 149(4):745–764
Illius AW, Gordon IJ (1987) The allometry of food intake in grazing ruminants. J Anim Ecol 56:989–999
Isvaran K (2005) Variation in male mating behaviour within ungulate populations: patterns and processes. Curr Sci 89:1192–1199
Isvaran K (2007) Intraspecific variation in group size in the blackbuck antelope: the roles of habitat structure and forage at different spatial scales. Oecology 154(2):435–444
Janis C (1976) The evolutionary strategy of the Equidae and the origins of rumen and cecal digestion. Evolution 30:757–776
Jarman P (1974) The social organisation of antelope in relation to their ecology. Behaviour 48(1–4):215–267
Jhala YV, Isvaran K (2016) Behavioural ecology of a grassland antelope, the blackbuck Antilope cervicapra: linking habitat, ecology and behaviour. In: The ecology of large herbivores in South and Southeast Asia. Springer, Dordrecht, pp 151–176
Krebs CJ (2001) Ecology. The experimental analysis of distribution and abundance. Benjamin Cummings, San Francisco
Kittle AM, Fryxell JM, Desy GE, Hamr J (2008) The scale-dependent impact of wolf predation risk on resource selection by three sympatric ungulates. Oecologia 157:163–175
Kuiters AT, Bruinderink GWG, Lammertsma DR (2005) Facilitative and competitive interactions between sympatric cattle, red deer and wild boar in Dutch woodland pastures. Acta Theriol 50(2):241–252
Kun-Rodrigues C, Jordi S, Aubin B, Emmanuel R, Clément R, Marques T, Lounès C (2014) New density estimates of a threatened sifaka species (Propithecus coquereli) in Ankarafantsika National Park. Am J Primatol 76(6):515–528
Lê S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat Soft 25(1):1–18
Long JL (2003) Introduced mammals of the world: their history distribution and influence. CSIRO Publication, Victoria, pp 486–487
Macandza VA, Owen-Smith N, Cain JW III (2012) Habitat and resource partitioning between abundant and relatively rare grazing ungulates. J Zool 287(3):175–185
Management Plan for Sathiyamangalam Wildlife Sanctuary (2010 to 2020) (2017) 1st ed. [ebook]. Tamil Nadu Forest Department, Chennai, pp 1–139. https://str-tn.org/wp-content/uploads/2014/07/Management-Plan-of-STR.pdf. Accessed 2 May 2017
Marques FFC, Buckland ST (2004) Covariate models for the detection function. In: Advanced distance sampling, pp 31–47.
Marques TA, Thomas L, Fancy SG, Buckland ST (2007) Improving estimates of bird density using multiple-covariate distance sampling. Auk 124(4):1229–1243
Marshall A, Lovett J, White P (2008) Selection of line-transect methods for estimating the density of group-living animals: lessons from the primates. Am J Primatol 70:452–462
Medici EP (2010) Assessing the viability of lowland tapir populations in a fragmented landscape. Ph.D. thesis, Kent University, London
Miller DL (2015) Distance: distance sampling detection function and abundance estimation. R package version 0.9, 3
Miller DL, Rexstad E, Thomas L, Marshall L, Laake JL (2019) Distance sampling in R. J Stat Soft 89(1):1–28
Mohr CO (1947) Table of equivalent populations of North American small mammals. Am Midl Nat 37(1):223–249
Mungall EC (1978) The Indian blackbuck antelope: a Texas view. Texas Agricultural Experiment, Texas, p 184
Murray MG, Illius AW (1996) Multi-species grazing in the Serengeti. In: Hodgson J, Illius AW (eds) The ecology and management of grazing systems. CAB International, Wallingford, pp 247–272
Murray MG, Illius AW (2000) Vegetation modification and resource competition in grazing ungulates. Oikos 89(3):501–508
Ogutu JO, Bhola N, Piepho HP, Reid R (2006) Efficiency of strip- and line-transect surveys of African savanna mammals. J Zool 269:149–160
Owen-Smith N (1982) Factors influencing the consumption of plant products by large herbivores. In: Ecology of tropical savannas. Springer, Berlin, pp 359–404
Pelliccioli F, Ferrari C (2014) The use of point-transects distance sampling to estimate the density of alpine marmot in the Gran Paradiso National Park. J Mount Ecol 9:47–60
Petrovan SO, Ward AI, Wheeler P (2011) Detectability counts when assessing populations for biodiversity targets. PLoS ONE 6(9):e24006
Prater SH (1948) The book of indian animals. Bombay Natural History Society, Bombay, p 314
Prins HHT, Olff H (1998) Species-richness of african grazer assemblages: towards a functional explanation. In: Dynamics of tropical communities: 37th Symposium of the British Ecological Society. Cambridge University Press, Cambridge, p 449
Putman RJ, Pratt R, Ekins J, Edwards PJ (1987) Food and feeding behaviour of cattle and ponies in the New Forest, Hampshire. J Appl Ecol 24:369–380
Ranjitsingh MK (1989) The Indian Blackbuck. Natraj Publishers, Dehradun, p 156
Regmi GR, Kandel K (2013) Estimating Group Density of Assamese Macaques (Macaca assamensis) using Multiple Covariate Distance Sampling (MCDS) in Lower Kanchenjungha Area (LKA), Estern Nepal. A Report submitted to the Primate Society of Great Britain
Rivera-Milán FF, Bertuol P, Simal F, Rusk BL (2015) Distance sampling survey and abundance estimation of the critically endangered Grenada Dove (Leptotila wellsi). The Condor 117(1):87–93
Robinson SK, Terborgh J (1995) Interspecific aggression and habitat selection by Amazonian birds. J Anim Ecol 64:1–11
Ross C, Reeve N (2003) Survey and census methods: population distribution and density. F. lab. Met. prim., pp 90–109.
Salter RE, Hudson RJ (1980) Range relationships of feral horses with wild ungulates and cattle in western Alberta. Range Ecol Manag 33(4):266–271
Schieltz JM, Rubenstein DI (2016) Evidence-based review: positive versus negative effects of livestock grazing on wildlife. What do we really know? Environ Res Lett 11:113003
Schmidt B (2003) Count data, detection probabilities, and the demography, dynamics, distribution, and decline of amphibians. C R Biol 326(Suppl 1):24
Silverman BW (1981) Using kernel density estimates to investigate multimodality. J R Stat Soc B 43(1):97–99
Smuts GL (1974) Age determination in Burchell’s zebra (Equus burchelli antiquorum) from the Kruger National Park. J S Afr Wildl Manag Assoc 4:103–115
Sukumar R, Venkataraman AB, Baskaran N, Dharmarajan G, Mukti R, Madhivanan A (2003) Resolving elephant-human conflicts in Asia—ecology of Asian elephant in Buxa Tiger Reserve and Jaldapara Wildlife Sanctuary (West Bengal) in relation to elephant-human conflict. A report to the Liz Claiborne and Art Ortenberg Foundation. Asian Elephant Research and Conservation Centre, Centre for Ecological Sciences, Indian Institute of Science, Bangalore
Thomas L, Buckland ST, Rexstad EA, Laake JL, Strindberg S, Hedley SL, Bishop JRB, Marques TA, Burnham KP (2010) Distance software: design and analysis of distance sampling surveys for estimating population size. J Appl Ecol 47:5–14. https://doi.org/10.1111/j.1365-2664.2009.01737.x
Wereszczuk A, Zalewski A (2015) Spatial niche segregation of sympatric stone marten and pine marten–avoidance of competition or selection of optimal habitat? PLoS ONE 10(10):e0139852
Williams R, Thomas L (2009) Cost-effective abundance estimation of rare animals: testing performance of small-boat surveys for killer whales in British Columbia. Biol Conserv 142:1542–1547
Williams BK, Nichols JD, Conroy MJ (2002) Analysis and management of animal populations: modelling, estimation, and decision making. Academic Press, San Diego
Zamboni T, Delgado A, Jiménez-Pérez I, De Angelo C (2015) How many are there? Multiple-covariate distance sampling for monitoring pampas deer in Corrientes, Argentina. Wildl Res 42(4):291–301
Zerbini AN (2006) Improving precision in multiple covariate distance sampling: a case study with Whales in Alaska. University of Washington, p 195
Acknowledgements
We acknowledge the Department of Science and Technology, Government of India, for funding this study under SERB Extramural Research Category. Our sincere thanks are also to the Tamil Nadu Forest Department, especially the former Chief Wildlife Warden, Mr. P.C. Tyagi, I.F.S., the present Chief Wildlife Warden, Mr. Sanjay Kumar Srivastava, I.F.S. and Chief Conservator of Forests, Sathiyamangalam Tiger Reserve, Erode, the Wildlife Wardens of Nagapattinam and Chennai, for granting permission and for logistic support. We also thank the management and the Principal of A.V.C. College for constant encouragement and support to this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all Co-Authors, the corresponding declares that we have no conflict of Interest.
Additional information
Handling editor: Luca Corlatti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arandhara, S., Sathishkumar, S. & Baskaran, N. Modelling the effect of covariates on the detectability and density of native blackbucks and invasive feral-horse using Multiple Covariate Distance Sampling at Point Calimere Wildlife Sanctuary, Southern India. Mamm Biol 100, 173–186 (2020). https://doi.org/10.1007/s42991-020-00018-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-020-00018-w