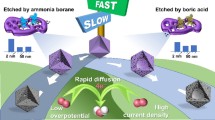
Abstract
We successfully synthesized a porous carbon material with abundant hexagonal boron nitride (h-BN) dispersed on a carbon matrix (p-BN-C) as efficient electrocatalysts for two-electron oxygen reduction reaction (2e− ORR) to produce hydrogen peroxide (H2O2). This catalyst was fabricated via ball-milling-assisted h-BN exfoliation and subsequent growth of carbon structure. In alkaline solutions, the h-BN/carbon heterostructure exhibited superior electrocatalytic activity for H2O2 generation measured by a rotating ring-disk electrode (RRDE), with a remarkable selectivity of up to 90–97% in the potential range of 0.3–0.6 V vs reversible hydrogen electrode (RHE), superior to most of the reported carbon-based electrocatalysts. Density functional theory (DFT) simulations indicated that the B atoms at the h-BN heterostructure interface were crucial active sites. These results underscore the remarkable catalytic activity of heterostructure and provide a novel approach for tailoring carbon-based catalysts, enhancing the selectivity and activity in the production of H2O2 through heterostructure engineering.





Similar content being viewed by others
Data and code availability
The data that support the findings of this study are available upon request from the corresponding author, upon reasonable request.
References
Sun Y, Han L, Strasser P (2020) A comparative perspective of electrochemical and photochemical approaches for catalytic H2O2 production. Chem Soc Rev 49:6605–6631. https://doi.org/10.1039/d0cs00458h
Shi X, Back S, Gill TM, Siahrostami S, Zheng X (2021) Electrochemical synthesis of H2O2 by two-electron water oxidation reaction. Chem 7:38–63. https://doi.org/10.1016/j.chempr.2020.09.013
Campos-Martin JM, Blanco-Brieva G, Fierro JL (2006) Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process. Angew Chem Int Ed 45:6962–6984. https://doi.org/10.1002/anie.200503779
Xu S, Lu R, Sun K, Tang J, Cen Y, Luo L, Wang Z, Tian S, Sun X (2022) Synergistic effects in N, O-comodified carbon nanotubes boost highly selective electrochemical oxygen reduction to H2O2. Adv Sci 9:e2201421. https://doi.org/10.1002/advs.202201421
Zhou Y, Chen G, Zhang J (2020) A review of advanced metal-free carbon catalysts for oxygen reduction reactions towards the selective generation of hydrogen peroxide. J Mater Chem A 8:20849–20869. https://doi.org/10.1039/d0ta07900f
Pizzutilo E, Kasian O, Choi CH, Cherevko S, Hutchings GJ, Mayrhofer KJJ, Freakley SJ (2017) Electrocatalytic synthesis of hydrogen peroxide on Au-Pd nanoparticles: from fundamentals to continuous production. Chem Phys Lett 683:436–442. https://doi.org/10.1016/j.cplett.2017.01.071
Slanac DA, Hardin WG, Johnston KP, Stevenson KJ (2012) Atomic ensemble and electronic effects in Ag-rich AgPd nanoalloy catalysts for oxygen reduction in alkaline media. J Am Chem Soc 134:9812–9819. https://doi.org/10.1021/ja303580b
Siahrostami S, Verdaguer-Casadevall A, Karamad M, Deiana D, Malacrida P, Wickman B, Escudero-Escribano M, Paoli EA, Frydendal R, Hansen TW, Chorkendorff I, Stephens IE, Rossmeisl J (2013) Enabling direct H2O2 production through rational electrocatalyst design. Nat Mater 12:1137–1143. https://doi.org/10.1038/nmat3795
Sheng H, Hermes ED, Yang X, Ying D, Janes AN, Li W, Schmidt JR, Jin S (2019) Electrocatalytic production of H2O2 by selective oxygen reduction using earth-abundant cobalt pyrite (CoS2). ACS Catal 9:8433–8442. https://doi.org/10.1021/acscatal.9b02546
Zhang L, Ren Y, Liu W, Wang A, Zhang T (2018) Single-atom catalyst: a rising star for green synthesis of fine chemicals. Nat Sci Rev 5:653–672. https://doi.org/10.1093/nsr/nwy077
Zhao Y, Raj J, Xu X, Jiang J, Wu J, Fan M (2024) Carbon catalysts empowering sustainable chemical synthesis via electrochemical CO2 conversion and two-electron oxygen reduction reaction. Small. https://doi.org/10.1002/smll.202311163
Zhang J-Y, Xia C, Wang H-F, Tang C (2022) Recent advances in electrocatalytic oxygen reduction for on-site hydrogen peroxide synthesis in acidic media. J Energy Chem 67:432–450. https://doi.org/10.1016/j.jechem.2021.10.013
Fan M, Wang Z, Sun K, Wang A, Zhao Y, Yuan Q, Wang R, Raj J, Wu J, Jiang J, Wang L (2023) N-B-OH site-activated graphene quantum dots for boosting electrochemical hydrogen peroxide production. Adv Mater 35:e2209086. https://doi.org/10.1002/adma.202209086
Yuan Q, Fan M, Zhao Y, Wu J, Raj J, Wang Z, Wang A, Sun H, Xu X, Wu Y, Sun K, Jiang J (2023) Facile fabrication of carbon dots containing abundant h-BN/graphite heterostructures as efficient electrocatalyst for hydrogen peroxide synthesis. Appl Catal B 324:122195. https://doi.org/10.1016/j.apcatb.2022.122195
Fan M, Wu J, Yuan J, Deng L, Zhong N, He L, Cui J, Wang Z, Behera SK, Zhang C, Lai J, Jawdat BI, Vajtai R, Deb P, Huang Y, Qian J, Yang J, Tour JM, Lou J, Chu CW, Sun D, Ajayan PM (2019) Doping nanoscale graphene domains improves magnetism in hexagonal boron nitride. Adv Mater 31:e1805778. https://doi.org/10.1002/adma.201805778
Shi Y, Hamsen C, Jia X, Kim KK, Reina A, Hofmann M, Hsu AL, Zhang K, Li H, Juang Z-Y, Dresselhaus MS, Li L-J, Kong J (2010) Synthesis of few-layer hexagonal boron nitride thin film by chemical vapor deposition. Nano Lett 10:4134–4139. https://doi.org/10.1021/nl1023707
Zhang Y, Lin Y, Duan T, Song L (2021) Interfacial engineering of heterogeneous catalysts for electrocatalysis. MaterToday 48:115–134. https://doi.org/10.1016/j.mattod.2021.02.004
Zhao Y, Xu X, Yuan Q, Wu Y, Sun K, Li B, Wang Z, Wang A, Sun H, Fan M, Jiang J (2023) Interfacial engineering of a vertically stacked graphene/h-BN heterostructure as an efficient electrocatalyst for hydrogen peroxide synthesis. Mater Horiz 10:4930–4939. https://doi.org/10.1039/d3mh00545c
Fan M, Wang Z, Zhao Y, Yuan Q, Cui J, Raj J, Sun K, Wang A, Wu J, Sun H, Li B, Wang L, Jiang J (2022) Porous heterostructure of graphene/hexagonal boron nitride as an efficient electrocatalyst for hydrogen peroxide generation. Carbon Energy 5:e309. https://doi.org/10.1002/cey2.309
Zhang T, Wang Y, Li X, Zhuang Q, Zhang Z, Zhou H, Ding Q, Wang Y, Dang Y, Duan L, Liu J (2023) Charge state modulation on boron site by carbon and nitrogen localized bonding microenvironment for two-electron electrocatalytic H2O2 production. Chin Chem Lett 34:107596. https://doi.org/10.1016/j.cclet.2022.06.019
Lei W, Portehault D, Dimova R, Antonietti M (2011) Boron carbon nitride nanostructures from salt melts: tunable water-soluble phosphors. J Am Chem Soc 133:7121–7127. https://doi.org/10.1021/ja200838c
Gao M, Wang Z-Y, Yuan Y-R, Li W-W, Liu H-Q, Huang T-Y (2022) Ball-milled biochar for efficient neutral electrosynthesis of hydrogen peroxide. Chem Eng J 434:134788. https://doi.org/10.1016/j.cej.2022.134788
Hod O (2012) Graphite and hexagonal boron-nitride have the same interlayer distance. Why? J Chem Theory Comput 8:1360–1369. https://doi.org/10.1021/ct200880m
Wang L, Wang Y, Xu T, Liao H, Yao C, Liu Y, Li Z, Chen Z, Pan D, Sun L, Wu M (2014) Gram-scale synthesis of single-crystalline graphene quantum dots with superior optical properties. Nat Commun 5:5357. https://doi.org/10.1038/ncomms6357
Fukamachi S, Solís-Fernández P, Kawahara K, Tanaka D, Otake T, Lin Y-C, Suenaga K, Ago H (2023) Large-area synthesis and transfer of multilayer hexagonal boron nitride for enhanced graphene device arrays. Nat Electron 6:126–136. https://doi.org/10.1038/s41928-022-00911-x
Wang S, Ye D, Liu H, Zhu X, Liu Z, Chen R, Liao Q, Yang Y (2022) Natural bamboo-derived O-doped rocky electrocatalyst for high-efficiency electrochemical reduction of O2 to H2O2. Int J Hydrogen Energy 47:5961–5973. https://doi.org/10.1016/j.ijhydene.2021.11.218
Zheng R, Meng Q, Zhang H, Li T, Yang D, Zhang L, Jia X, Liu C, Zhu J, Duan X, Xiao M, Xing W (2024) Atomically dispersed Fe sites on hierarchically porous carbon nanoplates for oxygen reduction reaction. J Energy Chem 90:7–15. https://doi.org/10.1016/j.jechem.2023.10.045
Fan M, Jimenez JD, Shirodkar SN, Wu J, Chen S, Song L, Royko MM, Zhang J, Guo H, Cui J, Zuo K, Wang W, Zhang C, Yuan F, Vajtai R, Qian J, Yang J, Yakobson BI, Tour JM, Lauterbach J, Sun D, Ajayan PM (2019) Atomic Ru immobilized on porous h-BN through simple vacuum filtration for highly active and selective CO2 methanation. ACS Catal 9:10077–10086. https://doi.org/10.1021/acscatal.9b02197
Wu Y, Yuan Q, Zhao Y, Xu X, Xu J, Wang Y, Sun K, Wang A, Sun H, Li B, Xu R, Wang Z, Jiang J, Fan M (2023) Boron-sulfur pairs for highly active 2e− oxygen reduction reaction to electrochemically synthesize hydrogen peroxide. ACS Sustain Chem Eng 11:13363–13373. https://doi.org/10.1021/acssuschemeng.3c02620
Li Q, Liu M, Zhang Y, Liu Z (2016) Hexagonal boron nitride-graphene heterostructures: synthesis and interfacial properties. Small 12:32–50. https://doi.org/10.1002/smll.201501766
Fan M, Zhu C, Yang J, Sun D (2016) Facile self-assembly N-doped graphene quantum dots/graphene for oxygen reduction reaction. Electrochim Acta 216:102–109. https://doi.org/10.1016/j.electacta.2016.09.014
Jung E, Shin H, Lee BH, Efremov V, Lee S, Lee HS, Kim J, Hooch Antink W, Park S, Lee KS, Cho SP, Yoo JS, Sung YE, Hyeon T (2020) Atomic-level tuning of Co-N-C catalyst for high-performance electrochemical H2O2 production. Nat Mater 19:436–442. https://doi.org/10.1038/s41563-019-0571-5
Lu Z, Chen G, Siahrostami S, Chen Z, Liu K, Xie J, Liao L, Wu T, Lin D, Liu Y, Jaramillo TF, Nørskov JK, Cui Y (2018) High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat Catal 1:156–162. https://doi.org/10.1038/s41929-017-0017-x
Li Z, Kumar A, Liu N, Cheng M, Zhao C, Meng X, Li H, Zhang Y, Liu Z, Zhang G, Sun X (2022) Oxygenated P/N co-doped carbon for efficient 2e- oxygen reduction to H2O2. J Mater Chem A 10:14355–14363. https://doi.org/10.1039/d2ta02590f
Xiang F, Zhao X, Yang J, Li N, Gong W, Liu Y, Burguete-Lopez A, Li Y, Niu X, Fratalocchi A (2022) Enhanced selectivity in the electroproduction of H2O2 via F/S dual-doping in metal-free nanofibers. Adv Mater 35:2207533. https://doi.org/10.1002/adma.202208533
Fan M, Xu J, Wang Y, Yuan Q, Zhao Y, Wang Z, Jiang J (2022) CO2 laser-induced graphene with an appropriate oxygen species as an efficient electrocatalyst for hydrogen peroxide synthesis. Chemistry 28:e202201996. https://doi.org/10.1002/chem.202201996
Deng Z, Gong M, Gong Z, Wang X (2022) Mesoscale mass transport enhancement on well-defined porous carbon platform for electrochemical H2O2 synthesis. Nano Lett 22:9551–9558. https://doi.org/10.1021/acs.nanolett.2c03696
Liu L, Kang L, Chutia A, Feng J, Michalska M, Ferrer P, Grinter DC, Held G, Tan Y, Zhao F, Guo F, Hopkinson DG, Allen CS, Hou Y, Gu J, Papakonstantinou I, Shearing PR, Brett DJL, Parkin IP, He G (2023) Spectroscopic identification of active sites of oxygen-doped carbon for selective oxygen reduction to hydrogen peroxide. Angew Chem Int Ed 62:e202303525. https://doi.org/10.1002/anie.202303525
Fan M, Yuan Q, Zhao Y, Wang Z, Wang A, Liu Y, Sun K, Wu J, Wang L, Jiang J (2022) A facile, “double-catalysts” approach to directionally fabricate pyridinic N‧B-pair-doped crystal graphene nanoribbons/amorphous carbon hybrid electrocatalysts for efficient oxygen reduction reaction. Adv Mater 34:2107040. https://doi.org/10.1002/adma.202107040
Ding G, Li C, Liu W, Zhao X, Jiang Y, Lu Y (2022) Enhanced H2O2 electrosynthesis on kneading oxidized carbon nanotubes. Appl Surf Sci 580:152293. https://doi.org/10.1016/j.apsusc.2021.152293
Watanabe K, Taniguchi T, Niiyama T, Miya K, Taniguchi M (2009) Far-ultraviolet plane-emission handheld device based on hexagonal boron nitride. Nat Photonics 3:591–594. https://doi.org/10.1038/nphoton.2009.167
Tian Q, Jing L, Chen Y, Su P, Tang C, Wang G, Liu J (2022) Micelle-templating interfacial self-assembly of two-dimensional mesoporous nanosheets for sustainable H2O2 electrosynthesis. Sustain Mater Technol 32:e00398. https://doi.org/10.1016/j.susmat.2022.e00398
Jiang K, Back S, Akey AJ, Xia C, Hu Y, Liang W, Schaak D, Stavitski E, Norskov JK, Siahrostami S, Wang H (2019) Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination. Nat Commun 10:3997. https://doi.org/10.1038/s41467-019-11992-2
Gasteiger HA, Kocha SS, Sompalli B, Wagner FT (2005) Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl Catal B 56:9–35. https://doi.org/10.1016/j.apcatb.2004.06.021
Abild-Pedersen F, Greeley J, Studt F, Rossmeisl J, Munter TR, Moses PG, Skulason E, Bligaard T, Norskov JK (2007) Scaling properties of adsorption energies for hydrogen-containing molecules on transition-metal surfaces. Phys Rev Lett 99:016105. https://doi.org/10.1103/PhysRevLett.99.016105
Lee D, Gan YX, Chen X, Kysar JW (2007) Influence of ultrasonic irradiation on the microstructure of Cu/Al2O3, CeO2 nanocomposite thin films during electrocodeposition. Mater Sci Eng 447:209–216. https://doi.org/10.1016/j.msea.2006.11.009
Peterson AA, Abild-Pedersen F, Studt F, Rossmeisl J, Nørskov JK (2010) How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ Sci 3:1311–1315. https://doi.org/10.1039/c0ee00071j
Acknowledgements
This work was supported by “National Natural Science Foundation of China (No. 32371810), the Foundation Research Project of Jiangsu Province (BK20221338), China Postdoctoral Science Foundation (No. 2023M731702), Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, International Innovation Center for Forest Chemicals and Materials, Nanjing Forestry University, merit-based funding for Nanjing innovation and technology project.
Author information
Authors and Affiliations
Contributions
XX has made significant contributions to this work. MMF designed the experiments. XX performed the major experiments and analyzed the results. QXY, YHW, and JWH carried out the electrochemical evaluations. YYZ conducted DFT calculations. XX wrote and revised the paper. MMF supervised the entire study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
42823_2024_718_MOESM1_ESM.docx
The experimental section. The SEM images of the p-BN-C and p-C. The HR-TEM images of the p-BN-C and pBN. The XPS data of the pBN. The collecting efficiency of H2O2. The retention rate of 26 mM H2O2 in 0.1 M KOH solution during 10 h at room temperature and out of light. The CV curves obtained for the p-BN-C, p-C, and p-B/N-C, the calculated ECSAs. Comparison of the catalytic performance between the p-BN-C and the recently reported electrocatalysts in an alkaline environment. The DFT-calculated data (DOCX 2198 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, X., Zhao, Y., Yuan, Q. et al. Porous heterostructure of h-BN/carbon as an efficient electrocatalyst for hydrogen peroxide generation. Carbon Lett. (2024). https://doi.org/10.1007/s42823-024-00718-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42823-024-00718-0