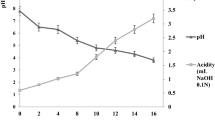
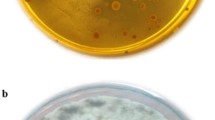
Abstract
This work aimed to characterize the antimicrobial compounds obtained from the potential probiotic Lactiplantibacillus plantarum S61, isolated from traditional fermented green olive, involved in their activity against fungi and bacteria responsible for food spoilage and poisonings. Their application as a biopreservative agent was also investigated. The culture of L. plantarum S61 showed substantial antifungal and antibacterial activity against yeasts (Rhodotorula glutinis and Candida pelliculosa), molds (Penicillium digitatum, Aspergillus niger, Fusarium oxysporum, and Rhizopus oryzae), and pathogenic bacteria (Listeria monocytogenes ATCC 19,117, Salmonella enterica subsp. enterica ATCC 14,028, Staphylococcus aureus subsp. aureus ATCC 6538, Pseudomonas aeruginosa ATCC 49,189), with inhibition zones > 10 mm. Likewise, the cell-free supernatant (CFS) of L. plantarum S61 showed an essential inhibitory effect against fungi and bacteria, with inhibition diameters of 12.25–22.05 mm and 16.95–17.25 mm, respectively. The CFS inhibited molds’ biomass and mycelium growth, with inhibition ranges of 63.18–83.64% and 22.57–38.93%, respectively. The antifungal activity of the CFS was stable during 4 weeks of storage at 25 °C, while it gradually decreased during storage at 4 °C. Several antimicrobial compounds were evidenced in the CFS of L. plantarum S61, including organic acids, ethanol, hydrogen peroxide, diacetyl, proteins, and fatty acids. The protein fraction, purified by reversed-phase high-performance liquid chromatography (RP-HPLC), demonstrated important antifungal activity, in relation to the fraction with molecular weight between 2 and 6 kDa. L. plantarum S61 and its CFS, tested in apple and orange fruit biopreservation, demonstrated their protective effect against P. digitatum spoilage. The CFS exhibited effectiveness in reducing Salmonella enterica subsp. enterica ATCC 14,028 in apple juice. L. plantarum S61 and/or its bioactive compounds CFS represent a promising strategy for biocontrol against pathogens and spoilage microorganisms in the agro-industry.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article.
References
FAO (2019) The state of food and agriculture: moving forward on food loss and waste reduction. http://www.fao.org/3/ca6030en/ca6030en.pdf. Accessed 20 March 2020
Pandey, A. and P.S. Negi (2018) Use of natural preservatives for shelf life extension of fruit juices. In: (ed) Fruit Juices Academic Press, pp 571–605
Moss MO (2008) Fungi, quality and safety issues in fresh fruits and vegetables. J Appl Microbiol 104:1239–1243. https://doi.org/10.1111/j.1365-2672.2007.03705.x
Salomão BdCM (2018) Pathogens and spoilage microorganisms in fruit juice. In: (ed) Fruit Juices, Academic Press, pp 291–308
Schnürer J, Magnusson J (2005) Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci Technol 16:70–78. https://doi.org/10.1016/j.tifs.2004.02.014
Leyva Salas M, Mounier J, Valence F, Coton M, Thierry A, Coton E (2017) Antifungal microbial agents for food biopreservation-a review. Microorganisms 5. https://doi.org/10.3390/microorganisms5030037
Tropcheva R, Nikolova D, Evstatieva Y, Danova S (2014) Antifungal activity and identification of Lactobacilli, isolated from traditional dairy product “katak.” Anaerobe 28:78–84. https://doi.org/10.1016/j.anaerobe.2014.05.010
Sadiq FA, Yan B, Tian F, Zhao J, Zhang H, Chen W (2019) Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: a comprehensive review. Compr Rev Food Sci Food Saf 18:1403–1436. https://doi.org/10.1111/1541-4337.12481
EFSA (2020) The 2019 updated list of QPS status recommended biological agents in support of EFSA risk assessments. EFSA J 18:5966
Dalié DKD, Deschamps AM, Richard-Forget F (2010) Lactic acid bacteria – potential for control of mould growth and mycotoxins: a review. Food Control 21:370–380. https://doi.org/10.1016/j.foodcont.2009.07.011
Magnusson J, Schnurer J (2001) Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl Environ Microbiol 67:1–5. https://doi.org/10.1128/AEM.67.1.1-5.2001
Niku-Paavola M-L, Laitila A, Mattila-Sandholm T, Haikara A (1999) New types of antimicrobial compounds produced by Lactobacillus plantarum. J Appl Microbiol. 86:29–35
Siedler S, Balti R, Neves AR (2019) Bioprotective mechanisms of lactic acid bacteria against fungal spoilage of food. Curr Opin Biotechnol 56:138–146. https://doi.org/10.1016/j.copbio.2018.11.015
Sjogren J, Magnusson J, Broberg A, Schnurer J, Kenne L (2003) Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl Environ Microbiol 69:7554–7557. https://doi.org/10.1128/aem.69.12.7554-7557.2003
Strom K, Sjogren J, Broberg A, Schnurer J (2002) Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(L-Phe-L-Pro) and cyclo(L-Phetrans-4-OH-L-Pro) and 3-phenyllactic acid. Appl Environ Microbiol 68:4322–4327
Trias R, Baneras L, Montesinos E, Badosa E (2008) Lactic acid bacteria from fresh fruit and vegetables as biocontrol agents of phytopathogenic bacteria and fungi. Int Microbiol 11:231–236. https://doi.org/10.2436/20.1501.01.66
Mora-Villalobos JA, Montero-Zamora J, Barboza N, Rojas-Garbanzo C, Usaga J, Redondo-Solano M, Schroedter L, Olszewska-Widdrat A, López-Gómez JP (2020) Multi-product lactic acid bacteria fermentations: a review. Fermentation 6:23. https://doi.org/10.3390/fermentation6010023
Arena MP, Silvain A, Normanno G, Grieco F, Drider D, Spano G, Fiocco D (2016) Use of Lactobacillus plantarum strains as a bio-control strategy against food-borne pathogenic microorganisms. Front Microbiol 7:464. https://doi.org/10.3389/fmicb.2016.00464
Abouloifa H, Rokni Y, Bellaouchi R, Ghabbour N, Karboune S, Brasca M, Salah RB, Chihib NE, Saalaoui E, Asehraou A (2020) Characterization of probiotic properties of antifungal Lactobacillus strains isolated from traditional fermenting green olives. Probiotics Antimicrob Proteins 12:683–696. https://doi.org/10.1007/s12602-019-09543-8
Abouloifa H, Gaamouche S, Rokni Y, Hasnaoui I, Bellaouchi R, Ghabbour N, Karboune S, Brasca M, D’Hallewin G, Ben Salah R, Saalaoui E, Asehraou A (2021) Antifungal activity of probiotic Lactobacillus strains isolated from natural fermented green olives and their application as food bio-preservative. Biol Control 152:104450. https://doi.org/10.1016/j.biocontrol.2020.104450
Zheng J, Wittouck S, Salvetti E, Franz C, Harris HMB, Mattarelli P, O’Toole PW, Pot B, Vandamme P, Walter J, Watanabe K, Wuyts S, Felis GE, Ganzle MG, Lebeer S (2020) A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol 70:2782–2858. https://doi.org/10.1099/ijsem.0.004107
Cavicchioli VQ, Camargo AC, Todorov SD, Nero LA (2017) Novel bacteriocinogenic Enterococcus hirae and Pediococcus pentosaceus strains with antilisterial activity isolated from Brazilian artisanal cheese. J Dairy Sci 100:2526–2535. https://doi.org/10.3168/jds.2016-12049
Gharbi Y, Fhoula I, Ruas-Madiedo P, Afef N, Boudabous A, Gueimonde M, Ouzari H-I (2018) In-vitro characterization of potentially probiotic Lactobacillus strains isolated from human microbiota: interaction with pathogenic bacteria and the enteric cell line HT29. Ann Microbiol 69:61–72. https://doi.org/10.1007/s13213-018-1396-1
Muhialdin BJ, Hassan Z (2011) Screening of lactic acid bacteria for antifungal activity against Aspergillus oryzae. Am J Appl Sci 8:447–451. https://doi.org/10.3844/ajassp.2011.447.451
Russo P, Arena MP, Fiocco D, Capozzi V, Drider D, Spano G (2017) Lactobacillus plantarum with broad antifungal activity: a promising approach to increase safety and shelf-life of cereal-based products. Int J Food Microbiol 247:48–54. https://doi.org/10.1016/j.ijfoodmicro.2016.04.027
A.O.A.C. (2000) Official Methods of Analysis. The Association of Official Analytical Chemists, Gaithersburg, MD, USA. 17th Edition
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Circle SJ, Stone L, Boruff C (1945) Acrolein determination by means of tryptophane. A colorimetric micromethod. Ind Eng Chem Anal Ed 17:259–262. https://doi.org/10.1021/i560140a021
Crowley S, Mahony J, van Sinderen D (2013) Broad-spectrum antifungal-producing lactic acid bacteria and their application in fruit models. Folia Microbiol 58:291–299. https://doi.org/10.1007/s12223-012-0209-3
Wang H, Sun Y, Chen C, Sun Z, Zhou Y, Shen F, Zhang H, Dai Y (2013) Genome shuffling of Lactobacillus plantarum for improving antifungal activity. Food Control 32:341–347. https://doi.org/10.1016/j.foodcont.2012.12.020
Nayyeri N, Edalatian Dovom MR, Habibi Najafi MB, Bahreini M (2017) A preliminary study on antifungal activity of lactic acid bacteria isolated from different production stages of Lighvan cheese on Penicillium expansum and Rhodotorula mucilaginosa. J Food Meas Charact 11:1734–1744. https://doi.org/10.1007/s11694-017-9554-x
Nallala V, Sadishkumar V, Jeevaratnam K (2017) Molecular characterization of antimicrobial Lactobacillus isolates and evaluation of their probiotic characteristics in vitro for use in poultry. Food Biotechnol 31:20–41. https://doi.org/10.1080/08905436.2016.1269289
Stanojević-Nikolić S, Dimić G, Mojović L, Pejin J, Djukić-Vuković A, Kocić-Tanackov S (2016) Antimicrobial activity of lactic acid against pathogen and spoilage microorganisms. J Food Process Preserv 40:990–998. https://doi.org/10.1111/jfpp.12679
Hof H (2019) Rhodotorula spp. in the gut - foe or friend? GMS Infect Dis 7:Doc02. https://doi.org/10.3205/id000042
Lin HC, Lin HY, Su BH, Ho MW, Ho CM, Lee CY, Lin MH, Hsieh HY, Lin HC, Li TC, Hwang KP, Lu JJ (2013) Reporting an outbreak of Candida pelliculosa fungemia in a neonatal intensive care unit. J Microbiol Immunol Infect 46:456–462. https://doi.org/10.1016/j.jmii.2012.07.013
Deepthi BV, Poornachandra Rao K, Chennapa G, Naik MK, Chandrashekara KT, Sreenivasa MY (2016) Antifungal attributes of Lactobacillus plantarum mys6 against fumonisin producing Fusarium proliferatum associated with poultry feeds. PLoS ONE 11:e0155122. https://doi.org/10.1371/journal.pone.0155122
Gerez CL, Torino MI, Rollán G, Font de Valdez G (2009) Prevention of bread mould spoilage by using lactic acid bacteria with antifungal properties. Food Control 20:144–148. https://doi.org/10.1016/j.foodcont.2008.03.005
Le Lay C, Coton E, Le Blay G, Chobert JM, Haertle T, Choiset Y, Van Long NN, Meslet-Cladiere L, Mounier J (2016) Identification and quantification of antifungal compounds produced by lactic acid bacteria and propionibacteria. Int J Food Microbiol 239:79–85. https://doi.org/10.1016/j.ijfoodmicro.2016.06.020
Salomskiene J, Jonkuviene D, Macioniene I, Abraitiene A, Zeime J, Repeckiene J, Vaiciulyte-Funk L (2019) Differences in the occurence and efficiency of antimicrobial compounds produced by lactic acid bacteria. Eur Food Res Technol 245:569–579. https://doi.org/10.1007/s00217-018-03227-3
Muhialdin BJ, Hassan Z, Saari N (2018) In vitro antifungal activity of lactic acid bacteria low molecular peptides against spoilage fungi of bakery products. Ann Microbiol 68:557–567. https://doi.org/10.1007/s13213-018-1363-x
Liu S, Ruan W, Li J, Xu H, Wang J, Gao Y, Wang J (2008) Biological control of phytopathogenic fungi by fatty acids. Mycopathologia 166:93–102. https://doi.org/10.1007/s11046-008-9124-1
Koba K, Yanagita T (2014) Health benefits of conjugated linoleic acid (CLA). Obes Res Clin Pract 8:e525–e532. https://doi.org/10.1016/j.orcp.2013.10.001
Rodrigues L, Banat IM, Teixeira J, Oliveira R (2006) Biosurfactants: potential applications in medicine. J Antimicrob Chemother 57:609–618. https://doi.org/10.1093/jac/dkl024
Santos DKF, Rufino RD, Luna JM, Santos VA, Sarubbo LA (2016) Biosurfactants: multifunctional biomolecules of the 21st century. Int J Mol Sci 17:401. https://doi.org/10.3390/ijms17030401
Sharma D, Saharan BS (2016) Functional characterization of biomedical potential of biosurfactant produced by Lactobacillus helveticus. Biotechnol Rep 11:27–35. https://doi.org/10.1016/j.btre.2016.05.001
Sharma D, Saharan BS, Shailly K (2016) Biosurfactants of lactic acid bacteria. Springer Switzerland
Degner BM, Chung C, Schlegel V, Hutkins R, McClements DJ (2014) Factors influencing the freeze-thaw stability of emulsion-based foods. Compr Rev Food Sci Food Saf 13:98–113. https://doi.org/10.1111/1541-4337.12050
Rouse S, Harnett D, Vaughan A, van Sinderen D (2008) Lactic acid bacteria with potential to eliminate fungal spoilage in foods. J Appl Microbiol 104:915–923. https://doi.org/10.1111/j.1365-2672.2007.03619.x
Barbosa AAT, Mantovani HC, Jain S (2017) Bacteriocins from lactic acid bacteria and their potential in the preservation of fruit products. Crit Rev Biotechnol 37:852–864. https://doi.org/10.1080/07388551.2016.1262323
Walker M, Phillips CA (2008) The effect of preservatives on Alicyclobacillus acidoterrestris and Propionibacterium cyclohexanicum in fruit juice. Food Control 19:974–981. https://doi.org/10.1016/j.foodcont.2007.10.003
Hatab S, Yue T, Mohamad O (2012) Removal of patulin from apple juice using inactivated lactic acid bacteria. J Appl Microbiol 112:892–899. https://doi.org/10.1111/j.1365-2672.2012.05279.x
Funding
The CNRST (PPR/19/2015), CNRST (Morocco)/CNRi (Italy) cooperation and McGill University provided financial support.
Author information
Authors and Affiliations
Contributions
HA and AA selected the study design. HA, IH, YR, RB, and SG conducted the experiments. HA and AA wrote the manuscript. SK, MB, GD, RBS, ES, NG, and AA performed review and editing. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abouloifa, H., Rokni, Y., Hasnaoui, I. et al. Characterization of antimicrobial compounds obtained from the potential probiotic Lactiplantibacillus plantarum S61 and their application as a biopreservative agent. Braz J Microbiol 53, 1501–1513 (2022). https://doi.org/10.1007/s42770-022-00791-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-022-00791-5