Abstract
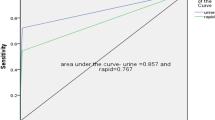
Tuberculosis remains one of the most important infectious diseases with well-known zoonotic nature that affect humans, wildlife, and domestic animals, including goats. Nonetheless, no intradermal tuberculin test has been standardized for caprine diagnosis of tuberculosis. The present study investigated the intradermal comparative cervical tuberculin test (ICCTT) in the diagnosis of tuberculosis among 60 goats from farms with history of tuberculosis. The cutoff applied to goats was based on a study where goats had been experimentally infected with Mycobacterium bovis and Mycobacterium avium. Clinical examination, bacteriological culture, and histopathological staining were assessed to the diagnosis. Isolates compatible with mycobacteria were subjected for molecular diagnosis based on gyrB-restriction fragment length polymorphism (RFLP) analysis and PCR restriction-enzyme analysis (PRA) of hsp65 gene by BstEII and HaeIII, namely PRA-hsp65 assay. From all goats, 60% (n = 36/60), 3.3% (n = 2/60), and 36.7% (n = 22/60) showed positive, inconclusive, and negative reactions, respectively. Out of 36 goats with ICCTT positive, 75% (n = 27/36) had isolation of mycobacteria and were detected M. bovis by gyrB-RFLP. Molecular diagnosis and histopathological findings compatible with tuberculosis showed 86.1% (n = 31/36) concordance with the ICCTT. When compared ICCTT with M. bovis isolation, gyrB-RFLP, and histopathology, the better arithmetic means of sensitivity and specificity were 2.5 mm for ICCTT compared with M. bovis isolation and gyrB-RFLP, and 4.55 mm when compared with histopathology. Both receiver operating characteristic (ROC) curves presented statistical significance (P < 0.001). The identification of other mycobacteria, e.g., M. kansasii, M. flavescens, M. avium, M. florentinum, M. lentiflavum, M. simiae, and Corynebacterium pseudotuberculosis, not influenced positive results in ICCTT. The concordance between bacteriological, histopathological, and molecular identification with ICCTT findings indicate that the tuberculin test may be used as a valuable tool for diagnosis of caprine tuberculosis and reinforce the importance of association of methods to diagnostic of the disease from animal origin.



Similar content being viewed by others
References
Pesciaroli M, Alvarez J, Boniotti MB, Cagiola M, Di Marco V, Marianelli C, Pacciarini M, Pasquali P (2014) Tuberculosis in domestic animal species. Res Vet Sci 97:S78–S85
Constable PD, Hinchliff KW, Done S, Gruenberg W (2016) Veterinary medicine: a textbook of the diseases of cattle, horses, sheep, pigs, and goats. 11th Edition, (Saunders, U.S.A.)
LoBue PA, Enarson DA, Thoen CO (2010) Tuberculosis in humans and animals: an overview. Int J Tuberc Lung Dis 14:1075–1078
Churchyard G, Kim P, Shah NS, Rustomjee R, Gandhi N, Mathema B, Dowdy D, Kasmar A, Cardena V (2017) What we know about tuberculosis transmission: an overview. J Infect Dis 216:629–635
Pandey GS, Hang’ombe BM, Mushabati F, Kataba A (2013) Prevalence of tuberculosis among southern Zambian cattle and isolation of Mycobacterium bovis in raw milk obtained from tuberculin positive cows. Vet World 6:986–991
Rastogi N, Legrand E, Sola C (2001) The mycobacteria: an introduction to nomenclature and pathogenesis. Rev Sci Tech 20:21–54
Alexander KA, Laver PN, Michael AL, Williams M, Van Helden PD, Warren RM, Van Pittius NCG (2010) Novel Mycobacterium tuberculosis complex pathogen, M mungi. EmergInfect Dis 16:1296–1299
Van Ingen J, Rahim Z, Mulder A, Boeree MJ, Simeone R, Brosch R, Van Soolinger D (2012) Characterization of Mycobacterium orygis as M. tuberculosis complex subspecies. Emerg Infect Dis 18:653–655
Dippener A, Parsons SDC, Sampson SL, Vander Merwe RG, Drewe JA, Abdallah AM, Siame KK, Van Pittius NCG, Van Helden PD, Pain A, Warren RM (2015) Whole genome sequence analysis of Mycobacterium suricattae. Tuberculosis 95:682–688
Somoskovi A, Salfinger M (2014) Nontuberculous mycobacteria in respiratory infections: advances in diagnosis and identification. Clin Labor Med 34:271–295
Stanford J, Stanford C (2012) Mycobacteria and their world. Intern J Mycobact 1:3–12
Daniel R, Evans H, Rolfe S, De La Rua DR, Crawshaw T, Higgins RJ, Schock A, Clifton-Hadley R (2009) Outbreak of tuberculosis caused by Mycobacterium bovis in golden Guernsey goats in Great Britain. Vet Rec 165:335–342
Crawshaw T, Daniel R, Clifton-Hadley R, Clark J, Evans H, Holfe S, De la Rua DR (2008) TB in goats caused by Mycobacterium bovis. Vet Rec 163:127
Seva J, Menchén V, Navarro JA, Pallarés FJ, Villar D, Vásquez F, Bernabé A (2002) Caprine tuberculosis eradication program: an immunohistochemical study. Small Rum Res 46:107–114
Melo LEH, Mota RA, Maia FCL, Fernandes ACC, Silva TIB, Leite JEB, Baptista Filho LCF, Ramos CAN (2012) Ocorrência e caracterização da tuberculose em caprinos leiteiros criados no estado de Pernambuco. Pesq Vet Bras 32:831–837
Benesi FJ, Pinheiro SR, Maiorka PC, Sakamoto SM, Roxo E, Benites NR, Birgel Junior EH, Gregory L (2008) Relato de caso: tuberculose em caprinos (Capra hircus). Arq Inst Biol 75:217–220
Pignata WA, Alves CJ, Azevedo SS, Dantas AFM, Gomes AAB, Remígio FR, Lima FS, Mota PM (2009) Prevalência para tuberculose caprina no semi-árido paraibano. Pesq Vet Bras 29:526–532
Erler W, Martin G, Sachse K, Maumann L, Kahlau D, Beer J, Bartos M, Nagy G, Cvetnic Z, Zolnir-Dovc M, Pavlik J (2004) Molecular fingerprinting of Mycobacterium bovis subsp. caprae isolates from Central Europe. J Clin Microbiol 42:2234–2238
Kassa GM, Abebe F, Worku Y, Legesse M, Medhin G, Bjune G, Ameni G (2012) Tuberculosis in goats and sheep in Afar Pastoral region of Ethiopia and isolation of Mycobacterium tuberculosis from goat. Vet Med Intern 2012:8
Barad DB, Chandel BS, Dadawala AI, Chauhan HC, Kher HS, Shorff S, Hagat AG, Singh SV, Singh PK, Sohal JS, Gupta S, Chaubey KK, Chakraborty S, Tiwari R, Deb R, Dhama K (2014) Incidence of Mycobacterium avium Subspecies paratuberculosis in Mehsani and Surti goats of Indian origin using multiple diagnosistic tests. J Biol Sci 14:124–133
Michelet L, Cruz K, Phalente Y, Karoui C, Henaut S, Beral M, Boschiroli ML (2016) Mycobacterium microti infection in goats, France. Emerg Infect Dis 22:569–570
World Organization for Animal Health (2019). Manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees). https://www.oie.int/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access/ Access 28 May 2021
Silva PEG, Pinheiro SR, Leal MLR, Bertagnon HGG, Motta PMPC, Sinhorini IL, Vasconcellos SA, Benesi FJ (2006) Teste de tuberculinização em caprinos (Capra hircus) experimentalmente sensibilizados. Ciência Rural 36:880–886
Centro Panamericano de Zoonosis. Organização Panamericana de la Salud. Manual de normas y procedimentos tecnicos para la bacteriologia de la tuberculosis. Buenos Aires: CEPANZO, 1988. 30p
Quinn PJ, Markey B K, Leonard FC, Fitzpatrick ES, Fanning S, Hartigan, PJ (2011) Veterinary microbiology and microbial disease. 2nd ed. Wiley-Blackwell, Chinchester, UK
Chimara E, Ferrazoli L, Leão SC (2004) Short Comunication: Mycobacterium tuberculosis Complex differentiation using gyrB-restrition fragment lenth polymorphism analysis. Mem Inst Oswaldo Cruz 99:745–748
Chimara E, Ferrazoli L, Ueky SYM, Martins MC, Durhan AM, Arbeit RD, Leão SC (2008) Reliable identification of Mycobacterial species by PCR-restrition enzyme analysis (PRA)-hsp65 in a reference laboratory and elaboration of a sequence-based extended algorithm of PRA-hsp65 patterns. BMC Microbiol 8:48
Thrusfield M, Christley R (2018) Veterinary epidemiology, 4th edn. Wiley, Oxford
Mackinnon A (2000) A spreads heet for calculation of comprehensive statistics for the assessment of diagnostic tests and inter-rater agreement. Comp Biol Med 30:127–134
Leite CQ, Anno IS, Leite SR, Roxo E, Morlock GP, Cooksey RC (2003) Isolation and identification of mycobacteria from livestock specimens and milk obtained in Brazil. Mem Inst Oswaldo Cruz 98:319–323
Franco MM, Paes AC, Ribeiro MG, Pantoja JC, Santos AC, Miyata M, Leite CQF, Motta RG, Listoni FJP (2013) Occurrence of mycobacteria in bovine milk samples from both individual and collective bulk tanks at farms and informal markets in the southeast region of Sao Paulo, Brazil. BMC Vet Res 9:85
Sgarioni SA, Hirata RD, Hirata MH, Leite CQ, Prince KA, Leite SR (2014) Occurrence of Mycobacterium bovis and non-tuberculous mycobacteria (NTM) in raw and pasteurized milk in the northwestern region of Paraná, Brazil. Braz J Microbiol 45:707–711
Johnson MM, Odell JA (2014) Nontuberculous mycobacterial pulmonary infections. J Thorac Dis 6:210–220
Agizew T, Surie D, Oeltmann JE, Letebele M, Pals S, Mathebula U, Mathona A, Kassa M, Hamda S, Pono P, Rankgoane-Pono G, Boyd R, Auld A, Finlay A (2020) Tuberculosis preventive treatment opportunities at antirretroviral therapy initiantion and follow-up visits. Public Health Act 10:64–69
Larsen AB (1952) The evaluation of acid-fast allergens on sensitized goats. Am J Vet Res 13:545–548
Thorel MF, Huchzermeyer W, Weiss R, Fontaine JJ (1997) Mycobacterium avium infections in animals. Literature Review. Vet Res 28:439–447
Bezos J, Álvarez J, Romero B, Aranaz A, de Juan L (2012) Tuberculosis in goats: assessment of current in vivo cell-mediated and antibody-based diagnostic assays. Vet J 191:161–165
Monaghan ML, Doherty ML, Collins JD, Kazda JF, Quinn PJ (1994) The tuberculin test. Vet Microbiol 40:111–124
Lilenbaum W (2000) Atualização em tuberculose bovina. Rev Bras Med Vet 22:145–151
Michel AL (2008) Mycobacterium fortuitum infection interference with Mycobacterium bovis diagnostics: natural infection cases and a pilot experimental infection. J Vet Diagn Invest 20:501–503
Bolanos CAD, Franco MMJ, Souza Filho AFS, Ikuta CYI, Burbano-Rosero EM, Ferreira Neto JS, Heinemann MB, Motta RG, de Paula CL, Morais ABC, Guerra ST, Alves AC, Listoni FJP, Ribeiro MG (2018) Nontuberculous mycobacteria in milk from positive cows in the intradermal comparative cervical tuberculin test: implications for human tuberculosis infections. Rev Inst Med Trop Sao Paulo 60:e6
Dorella FA, Pacheco LG, Oliveira SC, Miyoshi A, Azevedo V (2006) Corynebacterium pseudotuberculosis: microbiology, biochemical properties, pathogenesis and molecular studies of virulence. Vet Res 37:201–208
Zamprogna TO, Ribeiro D, Azevedo VAC, Lara GHB, Motta RG, Silva RC, Siqueira AK, Nardi Júnior G, Listoni FJP, Martins LSA, Silva AV, Portilho FVR, Mota ARM, Rodrigues CA, Almeida BO, Ribeiro MG (2021) Bacteriological, cytological, and molecular investigation of Corynebacterium pseudotuberculosis, mycobacteria, and other bacteria in caseous lymphadenitis and healthy lymph nodes of slaughtered sheep. Braz J Microbiol 52:431–438
Sarathy JP, Dartois V (2020) Caseum: a niche for Mycobaterium tuberculosis drug-tolerant persister. Clin Microbiol Rev 33:e00159–e219
Bernabé A, Gómez MA, Navarro JA, Gómez S, Sánchez J, Sidrach J, Menchén V (1991) Pathological changes of spontaneous dual infection of tuberculosis and paratuberculosis in goats. Small Rum Res 5:377–390
Cousins DV, Francis BR, Casey R, Mayberry C (1993) Mycobacterium bovis infection in a goat. Aust Vet J 70:262–263
Koneman EW, Allen SD, Ionda WM, Schreckenberger PC, Winn JRWC (2001) Diagnóstico Microbiológico: texto e atlas colorido. 5 ed. Medsi, RJ, Brasil
Acosta B, Real F, Ferrer O, Deniz S, Poveda JB (1998) Isolation of Mycobacterium kansasii from a tuberculin-positive goat. Vet Rec 142:195–196
Higino SSS, Pinheiro SR, Souza GO, Dib CC, Rosário TR, Melville PA, Alves CJ, Azevedo SS (2011) Mycobacterium bovis infection in goats from the northeaste region of Brazil. Braz J Microbiol 42:1437–1439
Lima e Silva WE, Gouveia GV, Sá MCA, Gouveia JJS, Peixoto RM, Riet-Correa F, Veschi JLA, Costa MM (2016) Bacteria isolated from abscesses of small ruminants inspected in the semiarid region of Brazil. Semina 37:1337–1344
Singh S, Viham V (2004) Detection of Mycobacterium avium subspecies paratuberculosis in goat milk. Small Rum Res 54:231–235
Kruze J, Salgado M, Paredes H, Mella A, Collins MT (2006) Goats paratuberculosis in Chile: first isolation and confirmation of Mycobacterium avium subspecies paratuberculosis infection in dairy goats. J Vet Diagn Invest 18:476–479
de Souza MCC, Lima MC, Braga IFE, Schwarz DGG, Rodrigues APS, Sales EB, Fonseca Júnior AA, Moreira MAS (2016) Molecular typing of Mycobacterium avium subsp. paratuberculosis (MAP) isolated from dairy goats in Brazil. Small Rum Res 140:18–21
To K, Cao R, Yegiazaryan A, Owens J, Venketaraman V (2020) General overview of nontuberculous mycobacteria opportunistic pathogens: Mycobacterium avium and Mycobacterium abscessus. J Clin Med 9:2541
Acknowledgements
We thank Josir Laine Aparecida Veschi, from Brazilian Agricultural Research Corporation (EMBRAPA) Semi-Árido, Petrolina, PE, Brazil, for his support in the fieldwork.
Funding
This study received support from São Paulo Research Foundation (FAPESP) by financial support (Process number 2006/58323–6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was performed in accordance with the Ethics Committee on Animal Use guidelines of the School of Veterinary Medicine and Animal Sciences of São Paulo State University-USP, São Paulo, SP, Brazil (protocol number 994/2006).
Conflict of the interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Miliane Moreira Soares de Souza
Rights and permissions
About this article
Cite this article
de Almeida, C.A.S., dos Santos, C.R., Benites, N.R. et al. Intradermal comparative cervical tuberculin test in the diagnosis of caprine tuberculosis. Braz J Microbiol 53, 421–431 (2022). https://doi.org/10.1007/s42770-021-00650-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00650-9