Abstract
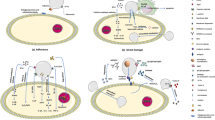
Histoplasma capsulatum is the causative agent of histoplasmosis, a systemic disease responsible for most reported causes of morbidity and mortality among immunosuppressed individuals. Peptidogalactomannan (pGM) was purified from the yeast cell wall of H. capsulatum isolated from bats, and its structure and involvement in modulating the host immune response were evaluated. Gas chromatography, methylation analysis, and two-dimensional nuclear magnetic resonance (2D-NMR) were used for the structural characterization of pGM. Methylation and 2D-NMR data revealed that pGM comprises a main chain containing α-d-Manp (1 → 6) residues substituted at O-2 by α-d-Manp (1 → 2)–linked side chains, non-reducing end units of α-d-Galf, or β-d-Galp linked (1→ 6) to α-d-Manp side chains. The involvement of H. capsulatum pGM in antigenic reactivity and in interactions with macrophages was demonstrated by ELISA and phagocytosis assay, respectively. The importance of the carbohydrate and protein moieties of pGM in sera reactivity was evaluated. Periodate oxidation abolished much pGM antigenic reactivity, suggesting that the sugar moiety is the most immunogenic part of pGM. Reactivity slightly decreased in pGM treated with proteinase K, suggesting that the peptide moiety plays a minor role in pGM antigenicity. In vitro experiments suggested that pGM is involved in the phagocytosis of H. capsulatum yeast and induction of IL-10 and IFN-γ secretion by peritoneal macrophages from C57BL/6 mice. These findings demonstrated the role of pGM in the H. capsulatum-host interaction.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Almeida MA, Almeida-Silva F, Guimarães AJ, Almeida-Paes R, Zancopé-Oliveira RM (2019) The occurrence of histoplasmosis in Brazil: a systematic review. Int J Infect Dis 86:147–156. https://doi.org/10.1016/j.ijid.2019.07.009
Kasuga T, White TJ, Koenig G, Mcewen J, Restrepo A, Castañeda E, Da Silva LC, Heins-Vaccari EM, De Freitas RS, Zancopé-Oliveira RM, Qin Z, Negroni R, Carter DA, Mikami Y, Tamura M, Taylor ML, Miller GF, Poonwan N, Taylor JW (2003) Phylogeography of the fungal pathogen Histoplasma capsulatum. Mol Ecol 12(12):3383–3401. https://doi.org/10.1046/j.1365-294X.2003.01995.x
Muniz MM, Silva ME, Tavares P, Meyer W, Nosanchuk JD, Zancope-Oliveira RM (2010) Comparison of different DNA-based methods for molecular typing of Histoplasma capsulatum. Appl Environ Microbiol 76(13):4438–4447. https://doi.org/10.1128/aem.02004-09
SEVERO LC, FdM OLIVEIRA, IRION K, NdS PORTO, LONDERO AT (2001) Histoplasmosis in Rio Grande do Sul, Brazil: a 21-year experience. Rev Inst Med Trop Sao Paulo 43:183–187
Latgé J-P, Beauvais A (2014) Functional duality of the cell wall. Curr Opin Microbiol 20:111–117. https://doi.org/10.1016/j.mib.2014.05.009
de Oliveira NF, Santos GRC, Xisto MIDS, Pires dos Santos GM, Nucci M, Haido RMT, Barreto-Bergter E (2018) β–1,6-linked Galactofuranose- rich peptidogalactomannan of Fusarium oxysporum is important in the activation of macrophage mechanisms and as a potential diagnostic antigen. Med Mycol 57(2):234–245. https://doi.org/10.1093/mmy/myx167
Haido RMT, Silva MH, Ejzemberg R, Leitão EA, Hearn VM, Evans EGV, Bergter EB (1998) Analysis of peptidogalactomannans from the mycelial surface of Aspergillus fumigatus. Med Mycol 36(5):313–321. https://doi.org/10.1080/02681219880000491
Lopes LCL, da Silva MID, Bittencourt VCB, Figueiredo RT, Rollin-Pinheiro R, Sassaki GL, Bozza MT, Gorin PAJ, Barreto-Bergter E (2011) Glycoconjugates and polysaccharides from the Scedosporium/Pseudallescheria boydii complex: structural characterisation, involvement in cell differentiation, cell recognition and virulence. Mycoses 54(s3):28–36. https://doi.org/10.1111/j.1439-0507.2011.02105.x
Lopes-Bezerra LM (2011) Sporothrix schenckii cell wall peptidorhamnomannans. Front Microbiol 2:243–243. https://doi.org/10.3389/fmicb.2011.00243
Xisto MIDS, Bittencourt VCB, Liporagi-Lopes LC, Haido RMT, Mendonça MSA, Sassaki G, Figueiredo RT, Romanos MTV, Barreto-Bergter E (2015) O-glycosylation in cell wall proteins in Scedosporium prolificans is critical for phagocytosis and inflammatory cytokines production by macrophages. PLoS One 10(4):e0123189–e0123189. https://doi.org/10.1371/journal.pone.0123189
Lloyd KO (1970) Isolation, characterization, and partial structure of peptido galactomannans from the yeast form of Cladosporium werneckii. Biochemistry 9(17):3446–3453. https://doi.org/10.1021/bi00819a025
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356. https://doi.org/10.1021/ac60111a017
Rondle CJ, Morgan WT (1955) The determination of glucosamine and galactosamine. Biochem J 61(4):586–589. https://doi.org/10.1042/bj0610586
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Kircher HW (1960) Gas-liquid partition chromatography of methylated sugars. Anal Chem 32(9):1103–1106. https://doi.org/10.1021/ac60165a016
Ciucanu I, Kerek F (1984) A simple and rapid method for the permethylation of carbohydrates. Carbohydr Res 131(2):209–217. https://doi.org/10.1016/0008-6215(84)85242-8
Santos GRC, Glauser BF, Parreiras LA, Vilanova E, Mourão PAS (2015) Distinct structures of the α-fucose branches in fucosylated chondroitin sulfates do not affect their anticoagulant activity. Glycobiology 25(10):1043–1052. https://doi.org/10.1093/glycob/cwv044
Lopes LCL, Rollin-Pinheiro R, Guimarães AJ, Bittencourt VCB, Martinez LR, Koba W, Farias SE, Nosanchuk JD, Barreto-Bergter E (2010) Monoclonal antibodies against peptidorhamnomannans of Scedosporium apiospermum enhance the pathogenicity of the fungus. PLoS Negl Trop Dis 4(10):e853. https://doi.org/10.1371/journal.pntd.0000853
Calixto R, Mattos B, Bittencourt V, Lopes L, Souza L, Sassaki G, Cipriani T, Silva M, Barreto-Bergter E (2010) β-Galactofuranose-containing structures present in the cell wall of the saprophytic fungus Cladosporium (Hormoconis) resinae. Res Microbiol 161(8):720–728. https://doi.org/10.1016/j.resmic.2010.07.005
Voller A, Bidwell DE, Bartlett A (1976) Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ 53(1):55–65
Tischer CA, Gorin PAJ, de Souza MB, Barreto-Bergter E (2002) Structures of phosphonogalactomannans isolated from mycelia of Aspergillus versicolor. Carbohydr Polym 49(2):225–230. https://doi.org/10.1016/S0144-8617(01)00325-3
Bittencourt VCB, Figueiredo RT, da Silva RB, Mourão-Sá DS, Fernandez PL, Sassaki GL, Mulloy B, Bozza MT, Barreto-Bergter E (2006) An α-glucan of Pseudallescheria boydii is involved in fungal phagocytosis and Toll-like receptor activation. J Biol Chem 281(32):22614–22623. https://doi.org/10.1074/jbc.M511417200
Ahrazem O, Prieto A, San-Blas G, Leal JA, Jiménez-Barbero J, Bernabé M (2003) Structural differences between the alkali-extracted water-soluble cell wall polysaccharides from mycelial and yeast phases of the pathogenic dimorphic fungus Paracoccidioides brasiliensis. Glycobiology 13(11):743–747. https://doi.org/10.1093/glycob/cwg069
Holbrook ED, Rappleye CA (2008) Histoplasma capsulatum pathogenesis: making a lifestyle switch. Curr Opin Microbiol 11(4):318–324. https://doi.org/10.1016/j.mib.2008.05.010
Antinori S (2014) Histoplasma capsulatum: more widespread than previously thought. Am J Trop Med Hyg 90(6):982–983. https://doi.org/10.4269/ajtmh.14-0175
Ferreira MS, Borges AS (2009) Histoplasmose. Rev Soc Bras Med Trop 42:192–198
Aidé MA (2009) Capítulo 4: histoplasmose. J Bras Pneumol 35:1145–1151
Assi M, Lakkis IE, Wheat LJ (2011) Cross-reactivity in the Histoplasma antigen enzyme immunoassay caused by sporotrichosis. Clin Vaccine Immunol 18(10):1781–1782. https://doi.org/10.1128/CVI.05017-11
Vergidis P, Walker RC, Kaul DR, Kauffman CA, Freifeld AG, Slagle DC, Kressel AB, Wheat LJ (2012) False-positive Aspergillus galactomannan assay in solid organ transplant recipients with histoplasmosis. Transpl Infect Dis 14(2):213–217. https://doi.org/10.1111/j.1399-3062.2011.00675.x
Xavier MO, Oliveira FM, Severo LC (2009) Capítulo 1: diagnóstico laboratorial das micoses pulmonares. J Bras Pneumol 35:907–919
Edwards JA, Kemski MM, Rappleye CA (2013) Identification of an aminothiazole with antifungal activity against intracellular Histoplasma capsulatum. Antimicrob Agents Chemother 57(9):4349–4359. https://doi.org/10.1128/AAC.00459-13
Guimarães AJ, de Cerqueira MD, Nosanchuk JD (2011) Surface architecture of histoplasma capsulatum. Front Microbiol 2:225–225. https://doi.org/10.3389/fmicb.2011.00225
Pinto MR, Barreto-Bergter E, Taborda CP (2008) Glycoconjugates and polysaccharides of fungal cell wall and activation of immune system. Braz J Microbiol 39(2):195–208. https://doi.org/10.1590/S1517-83822008000200001
Pinto MR, de Sá ACM, Limongi CL, Rozental S, Santos ALS, Barreto-Bergter E (2004) Involvement of peptidorhamnomannan in the interaction of Pseudallescheria boydii and HEp2 cells. Microbes Infect 6(14):1259–1267. https://doi.org/10.1016/j.micinf.2004.07.006
Tronchin G, Pihet M, Lopes-Bezerra LM, Bouchara J-P (2008) Adherence mechanisms in human pathogenic fungi. Med Mycol 46(8):749–772. https://doi.org/10.1080/13693780802206435
Barreto-Bergter E, Figueiredo RT (2014) Fungal glycans and the innate immune recognition. Front Cell Infect Microbiol 4:145–145. https://doi.org/10.3389/fcimb.2014.00145
Roilides E, Simitsopoulou M, Katragkou A, Walsh TJ (2009) Host immune response against Scedosporium species. Med Mycol 47(4):433–440. https://doi.org/10.1080/13693780902738006
Azuma I, Kanetsuna F, Tanaka Y, Yamamura Y, Carbonell LM (1974) Chemical and immunological properties of galactomannans obtained from Histoplasma duboisii, Histoplasma capsulatum, Paracoccidioides brasiliensis and Blasomyces dermatitidis. Mycopathol Mycol Appl 54(1):111–125. https://doi.org/10.1007/BF02055979
Kanetsuna F, Carbonell LM, Gil F, Azuma I (1974) Chemical and ultrastructural studies on the cell walls of the yeastlike and mycelial forms of Histoplasma capsulatum. Mycopathol Mycol Appl 54(1):1–13. https://doi.org/10.1007/BF02055967
Reiss E, Mitchell WO, Stone SH, Hasenclever HF (1974) Cellular immune activity of a galactomannan-protein complex from mycelia of Histoplasma capsulatum. Infect Immun 10(4):802–809
Leitão EA, Bittencourt VCB, Haido RMT, Valente AP, Peter-Katalinic J, Letzel M, de Souza LM, Barreto-Bergter E (2003) β-Galactofuranose-containing O-linked oligosaccharides present in the cell wall peptidogalactomannan of Aspergillus fumigatus contain immunodominant epitopes. Glycobiology 13(10):681–692. https://doi.org/10.1093/glycob/cwg089
Flavia Popi A, Daniel Lopes J, Mariano M (2002) GP43 from Paracoccidioides brasiliensis inhibits macrophage functions. An evasion mechanism of the fungus. Cell Immunol 218(1):87–94. https://doi.org/10.1016/S0008-8749(02)00576-2
Konno FTC, Maricato J, Konno AYC, Guereschi MG, Vivanco BC, Feitosa LS, Mariano M, Lopes JD (2012) Paracoccidioides brasiliensis GP43-derived peptides are potent modulators of local and systemic inflammatory response. Microbes Infect 14(6):517–527. https://doi.org/10.1016/j.micinf.2011.12.012
Latgé JP (1999) Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev 12(2):310–350
Kroetz DN, Deepe GS (2012) The role of cytokines and chemokines in Histoplasma capsulatum infection. Cytokine 58(1):112–117. https://doi.org/10.1016/j.cyto.2011.07.430
Hazlett LD, Jiang X, McClellan SA (2014) IL-10 function, regulation, and in bacterial keratitis. J Ocul Pharmacol Ther 30(5):373–380. https://doi.org/10.1089/jop.2014.0018
Figueiredo RT, Fernandez PL, Dutra FF, González Y, Lopes LC, Bittencourt VCB, Sassaki GL, Barreto-Bergter E, Bozza MT (2010) TLR4 recognizes Pseudallescheria boydii conidia and purified rhamnomannans. J Biol Chem 285(52):40714–40723. https://doi.org/10.1074/jbc.M110.181255
Sahaza JH, Suárez-Alvarez R, Estrada-Bárcenas DA, Pérez-Torres A, Taylor ML (2015) Profile of cytokines in the lungs of BALB/c mice after intra-nasal infection with Histoplasma capsulatum mycelial propagules. Comp Immunol Microbiol Infect Dis 41:1–9. https://doi.org/10.1016/j.cimid.2015.05.003
Acknowledgments
We thank Paulo A. S. Mourão for the advice, encouragement, and support of this manuscript. We also thank Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)-Financial Code 001, Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq), and Universidade Federal Fluminense (UFF).
Code availability
Not applicable.
Funding
This study was funded by Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ) (grant number E-26/200.577/2016). This study was partly funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (Financial Code 001) and Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Contributions
GMPS, MIDSX, RR-P, and MRP designed the experiments and drafted the manuscript. GMPS, GRCS, MIDSX, and RR-P performed all experiments. GMPS, GRCS, MIDSX, RR-P, ARSB, EMSR, RLDM, EB-B, and MRP analyzed the data. EB-B critically revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the research ethics committee of Instituto Nacional de Infectologia Evandro Chagas, Fiocruz, accession number 19109913.0.0000.5262, and by the Institutional Animal Welfare Committee of the Universidade Federal Fluminense (UFF), accession code 731/2016/CEUA-UFF.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Responsible Editor: Rosana Puccia
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
dos Santos, G.M.P., dos Santos, G.R.C., Xisto, M.I.D.d. et al. Peptidogalactomannan from Histoplasma capsulatum yeast cell wall: role of the chemical structure in recognition and activation by peritoneal macrophages. Braz J Microbiol 52, 479–489 (2021). https://doi.org/10.1007/s42770-021-00447-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00447-w