Abstract
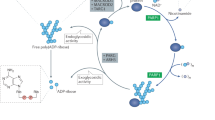
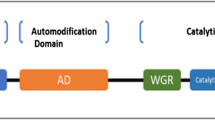
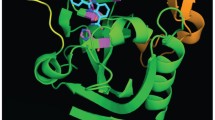
Poly(ADP-ribosyl)ation (PARylation), a type of post-translational modification catalyzed by poly(ADP-ribose) polymerase (PARP), is implicated in numerous biological processes including DNA repair, chromatin remodeling, programmed cell death, RNA regulation, and PAR-dependent ubiquitination. The advent of PARP inhibitors represents a new synthetic lethality paradigm for killing tumors bearing BRCA mutations in which tumor-specific defects are exploited to create a vulnerability that causes tumor cell death. To date, four PARP inhibitors have been approved by the US Food and Drug Administration for treatment of several types of cancer. In this review, we summarize the current knowledge of the molecular functions of PARP1 and highlight the recent advances in the use of PARP inhibitors in cancer treatment and the problem of drug resistance.



Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Adamowicz, M., Hailstone, R., Demin, A. A., Komulainen, E., Hanzlikova, H., Brazina, J., Gautam, A., Wells, S. E., & Caldecott, K. W. (2021). XRCC1 protects transcription from toxic PARP1 activity during DNA base excision repair. Nature Cell Biology, 23(12), 1287–1298. https://doi.org/10.1038/s41556-021-00792-w
Alano, C. C., Garnier, P., Ying, W., Higashi, Y., Kauppinen, T. M., & Swanson, R. A. (2010). NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. The Journal of Neuroscience, 30(8), 2967–2978. https://doi.org/10.1523/JNEUROSCI.5552-09.2010
Alemasova, E. E., & Lavrik, O. I. (2019). Poly(ADP-ribosyl)ation by PARP1: Reaction mechanism and regulatory proteins. Nucleic Acids Research, 47(8), 3811–3827. https://doi.org/10.1093/nar/gkz120
Alvarez-Gonzalez, R., & Mendoza-Alvarez, H. (1995). Dissection of ADP-ribose polymer synthesis into individual steps of initiation, elongation, and branching. Biochimie, 77(6), 403–407. https://doi.org/10.1016/0300-9084(96)88153-3
Amé, J.-C., Fouquerel, E., Gauthier, L. R., Biard, D., Boussin, F. D., Dantzer, F., de Murcia, G., & Schreiber, V. (2009). Radiation-induced mitotic catastrophe in PARG-deficient cells. Journal of Cell Science, 122(Pt 12), 1990–2002. https://doi.org/10.1242/jcs.039115
Amé, J.-C., Rolli, V., Schreiber, V., Niedergang, C., Apiou, F., Decker, P., Muller, S., Höger, T., Murcia, J. M., & de Murcia, G. (1999). PARP-2, A novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase*. Journal of Biological Chemistry, 274(25), 17860–17868. https://doi.org/10.1074/jbc.274.25.17860
Anderson, P., & Kedersha, N. (2008). Stress granules: The Tao of RNA triage. Trends in Biochemical Sciences, 33(3), 141–150. https://doi.org/10.1016/j.tibs.2007.12.003
Andrei, L., Kasas, S., Ochoa Garrido, I., Stanković, T., Suárez Korsnes, M., Vaclavikova, R., Assaraf, Y. G., & Pešić, M. (2020). Advanced technological tools to study multidrug resistance in cancer. Drug Resistance Updates, 48, 100658. https://doi.org/10.1016/j.drup.2019.100658
Barazas, M., Annunziato, S., Pettitt, S. J., de Krijger, I., Ghezraoui, H., Roobol, S. J., Lutz, C., Frankum, J., Song, F. F., Brough, R., Evers, B., Gogola, E., Bhin, J., van de Ven, M., van Gent, D. C., Jacobs, J. J. L., Chapman, R., Lord, C. J., Jonkers, J., & Rottenberg, S. (2018). The CST complex mediates end protection at double-strand breaks and promotes PARP inhibitor sensitivity in BRCA1-deficient cells. Cell Reports, 23(7), 2107–2118. https://doi.org/10.1016/j.celrep.2018.04.046
Barber, L. J., Sandhu, S., Chen, L., Campbell, J., Kozarewa, I., Fenwick, K., Assiotis, I., Rodrigues, D. N., Reis Filho, J. S., Moreno, V., Mateo, J., Molife, L. R., De Bono, J., Kaye, S., Lord, C. J., & Ashworth, A. (2013). Secondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitor. The Journal of Pathology, 229(3), 422–429. https://doi.org/10.1002/path.4140
Beck, C., Boehler, C., Guirouilh Barbat, J., Bonnet, M.-E., Illuzzi, G., Ronde, P., Gauthier, L. R., Magroun, N., Rajendran, A., Lopez, B. S., Scully, R., Boussin, F. D., Schreiber, V., & Dantzer, F. (2014). PARP3 affects the relative contribution of homologous recombination and nonhomologous end-joining pathways. Nucleic Acids Research, 42(9), 5616–5632. https://doi.org/10.1093/nar/gku174
Bilokapic, S., Suskiewicz, M. J., Ahel, I., & Halic, M. (2020). Bridging of DNA breaks activates PARP2–HPF1 to modify chromatin. Nature, 585(7826), 609–613. https://doi.org/10.1038/s41586-020-2725-7
Boamah, E. K., Kotova, E., Garabedian, M., Jarnik, M., & Tulin, A. V. (2012). Poly(ADP-Ribose) POLYMERASE 1 (PARP-1) regulates ribosomal biogenesis in drosophila nucleoli. PLoS Genetics, 8(1), e1002442. https://doi.org/10.1371/journal.pgen.1002442
Bock, F. J., Todorova, T. T., & Chang, P. (2015). RNA Regulation by poly(ADP-Ribose) polymerases. Molecular Cell, 58(6), 959–969. https://doi.org/10.1016/j.molcel.2015.01.037
Boehler, C., Gauthier, L. R., Mortusewicz, O., Biard, D. S., Saliou, J.-M., Bresson, A., Sanglier-Cianferani, S., Smith, S., Schreiber, V., Boussin, F., & Dantzer, F. (2011). Poly(ADP-ribose) polymerase 3 (PARP3), a newcomer in cellular response to DNA damage and mitotic progression. Proceedings of the National Academy of Sciences, 108(7), 2783–2788. https://doi.org/10.1073/pnas.1016574108
Brochu, G., Duchaine, C., Thibeault, L., Lagueux, J., Shah, G. M., & Poirier, G. G. (1994). Mode of action of poly(ADP-ribose) glycohydrolase. Biochimica Et Biophysica Acta, 1219(2), 342–350. https://doi.org/10.1016/0167-4781(94)90058-2
Bryant, H. E., Schultz, N., Thomas, H. D., Parker, K. M., Flower, D., Lopez, E., Kyle, S., Meuth, M., Curtin, N. J., & Helleday, T. (2005). Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature, 434(7035), 913–917. https://doi.org/10.1038/nature03443
Bunting, S. F., Callén, E., Wong, N., Chen, H.-T., Polato, F., Gunn, A., Bothmer, A., Feldhahn, N., Fernandez-Capetillo, O., Cao, L., Xu, X., Deng, C.-X., Finkel, T., Nussenzweig, M., Stark, J. M., & Nussenzweig, A. (2010). 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection OF DNA breaks. Cell, 141(2), 243–254. https://doi.org/10.1016/j.cell.2010.03.012
Calabrese, C. R., Almassy, R., Barton, S., Batey, M. A., Calvert, A. H., Canan-Koch, S., Durkacz, B. W., Hostomsky, Z., Kumpf, R. A., Kyle, S., Li, J., Maegley, K., Newell, D. R., Notarianni, E., Stratford, I. J., Skalitzky, D., Thomas, H. D., Wang, L.-Z., Webber, S. E., & Curtin, N. J. (2004). Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. Journal of the National Cancer Institute, 96(1), 56–67. https://doi.org/10.1093/jnci/djh005
Caldecott, K. W., Aoufouchi, S., Johnson, P., & Shall, S. (1996). XRCC1 polypeptide interacts with DNA polymerase beta and possibly poly (ADP-ribose) polymerase, and DNA ligase III is a novel molecular “nick-sensor” in vitro. Nucleic Acids Research, 24(22), 4387–4394. https://doi.org/10.1093/nar/24.22.4387
Caldecott, K. W., McKeown, C. K., Tucker, J. D., Ljungquist, S., & Thompson, L. H. (1994). An interaction between the mammalian DNA repair protein XRCC1 and DNA ligase III. Molecular and Cellular Biology, 14(1), 68–76. https://doi.org/10.1128/mcb.14.1.68-76.1994
Ceccaldi, R., Liu, J. C., Amunugama, R., Hajdu, I., Primack, B., Petalcorin, M. I. R., O’Connor, K. W., Konstantinopoulos, P. A., Elledge, S. J., Boulton, S. J., Yusufzai, T., & D’Andrea, A. D. (2015). Homologous-recombination-deficient tumours are dependent on Polθ-mediated repair. Nature, 518(7538), 258–262. https://doi.org/10.1038/nature14184
Ceccaldi, R., Rondinelli, B., & D’Andrea, A. D. (2016). Repair pathway choices and consequences at the double-strand break. Trends in Cell Biology, 26(1), 52–64. https://doi.org/10.1016/j.tcb.2015.07.009
Chambon, P., Weill, J. D., & Mandel, P. (1963). Nicotinamide mononucleotide activation of new DNA-dependent polyadenylic acid synthesizing nuclear enzyme. Biochemical and Biophysical Research Communications, 11, 39–43. https://doi.org/10.1016/0006-291x(63)90024-x
Chen, C.-C., Feng, W., Lim, P. X., Kass, E. M., & Jasin, M. (2018a). Homology-directed repair and the role of BRCA1, BRCA2, and related proteins in genome integrity and cancer. Annual Review of Cancer Biology, 2, 313–336. https://doi.org/10.1146/annurev-cancerbio-030617-050502
Chen, Q., Kassab, M. A., Dantzer, F., & Yu, X. (2018b). PARP2 mediates branched poly ADP-ribosylation in response to DNA damage. Nature Communications. https://doi.org/10.1038/s41467-018-05588-5
Chiu, L.-Y., Ho, F.-M., Shiah, S.-G., Chang, Y., & Lin, W.-W. (2011). Oxidative stress initiates DNA damager MNNG-induced poly(ADP-ribose)polymerase-1-dependent parthanatos cell death. Biochemical Pharmacology, 81(3), 459–470. https://doi.org/10.1016/j.bcp.2010.10.016
Clarke, N., Wiechno, P., Alekseev, B., Sala, N., Jones, R., Kocak, I., Chiuri, V. E., Jassem, J., Fléchon, A., Redfern, C., Goessl, C., Burgents, J., Kozarski, R., Hodgson, D., Learoyd, M., & Saad, F. (2018). Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: A randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet Oncology, 19(7), 975–986. https://doi.org/10.1016/S1470-2045(18)30365-6
Coleman, R. L., Fleming, G. F., Brady, M. F., Swisher, E. M., Steffensen, K. D., Friedlander, M., Okamoto, A., Moore, K. N., Efrat Ben-Baruch, N., Werner, T. L., Cloven, N. G., Oaknin, A., DiSilvestro, P. A., Morgan, M. A., Nam, J.-H., Leath, C. A., Nicum, S., Hagemann, A. R., Littell, R. D., & Bookman, M. A. (2019). Veliparib with first-line chemotherapy and as Maintenance therapy in ovarian cancer. New England Journal of Medicine, 381(25), 2403–2415. https://doi.org/10.1056/NEJMoa1909707
Coleman, R. L., Oza, A. M., Lorusso, D., Aghajanian, C., Oaknin, A., Dean, A., Colombo, N., Weberpals, J. I., Clamp, A., Scambia, G., Leary, A., Holloway, R. W., Gancedo, M. A., Fong, P. C., Goh, J. C., O’Malley, D. M., Armstrong, D. K., Garcia-Donas, J., Swisher, E. M., ARIEL3 investigators. (2017). Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (london, England), 390(10106), 1949–1961. https://doi.org/10.1016/S0140-6736(17)32440-6
Cropper, J. D., Alimbetov, D. S., Brown, K. T. G., Likhotvorik, R. I., Robles, A. J., Guerra, J. T., He, B., Chen, Y., Kwon, Y., & Kurmasheva, R. T. (2022). PARP1-MGMT complex underpins pathway crosstalk in O6-methylguanine repair. Journal of Hematology & Oncology, 15(1), 146. https://doi.org/10.1186/s13045-022-01367-4
DaRosa, P. A., Wang, Z., Jiang, X., Pruneda, J. N., Cong, F., Klevit, R. E., & Xu, W. (2015). Allosteric activation of the RNF146 ubiquitin ligase by a poly(ADP-ribosyl)ation signal. Nature, 517(7533), 223–226. https://doi.org/10.1038/nature13826
Daugas, E., Susin, S. A., Zamzami, N., Ferri, K. F., Irinopoulou, T., Larochette, N., Prévost, M. C., Leber, B., Andrews, D., Penninger, J., & Kroemer, G. (2000). Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB Journal, 14(5), 729–739.
Day, T. A., Layer, J. V., Cleary, J. P., Guha, S., Stevenson, K. E., Tivey, T., Kim, S., Schinzel, A. C., Izzo, F., Doench, J., Root, D. E., Hahn, W. C., Price, B. D., & Weinstock, D. M. (2017). PARP3 is a promoter of chromosomal rearrangements and limits G4 DNA. Nature Communications. https://doi.org/10.1038/ncomms15110
Del Campo, J. M., Matulonis, U. A., Malander, S., Provencher, D., Mahner, S., Follana, P., Waters, J., Berek, J. S., Woie, K., Oza, A. M., Canzler, U., Gil-Martin, M., Lesoin, A., Monk, B. J., Lund, B., Gilbert, L., Wenham, R. M., Benigno, B., Arora, S., & Mirza, M. R. (2019). Niraparib maintenance therapy in patients with recurrent ovarian cancer after a partial response to the last platinum-based chemotherapy in the ENGOT-OV16/NOVA trial. Journal of Clinical Oncology, 37(32), 2968–2973. https://doi.org/10.1200/JCO.18.02238
Demple, B., & Harrison, L. (1994). Repair of oxidative damage to DNA: Enzymology and biology. Annual Review of Biochemistry, 63, 915–948. https://doi.org/10.1146/annurev.bi.63.070194.004411
Dias, M. P., Moser, S. C., Ganesan, S., & Jonkers, J. (2021). Understanding and overcoming resistance to PARP inhibitors in cancer therapy. Nature Reviews Clinical Oncology, 18(12), 773–791. https://doi.org/10.1038/s41571-021-00532-x
Drew, Y., Kaufman, B., Banerjee, S., Lortholary, A., Hong, S. H., Park, Y. H., Zimmermann, S., Roxburgh, P., Ferguson, M., Alvarez, R. H., Domchek, S., Gresty, C., Angell, H. K., Ros, V. R., Meyer, K., Lanasa, M., Herbolsheimer, P., & de Jonge, M. (2019). 1190PD - phase II study of olaparib + durvalumab (MEDIOLA): Updated results in germline BRCA-mutated platinum-sensitive relapsed (PSR) ovarian cancer (OC). Annals of Oncology, 30, v485–v486. https://doi.org/10.1093/annonc/mdz253.016
Drew, Y., Ledermann, J., Hall, G., Rea, D., Glasspool, R., Highley, M., Jayson, G., Sludden, J., Murray, J., Jamieson, D., Halford, S., Acton, G., Backholer, Z., Mangano, R., Boddy, A., Curtin, N., & Plummer, R. (2016). Phase 2 multicentre trial investigating intermittent and continuous dosing schedules of the poly(ADP-ribose) polymerase inhibitor rucaparib in germline BRCA mutation carriers with advanced ovarian and breast cancer. British Journal of Cancer, 114(12), e21. https://doi.org/10.1038/bjc.2016.133
Dungrawala, H., Bhat, K. P., Le Meur, R., Chazin, W. J., Ding, X., Sharan, S. K., Wessel, S. R., Sathe, A. A., Zhao, R., & Cortez, D. (2017). RADX promotes genome stability and modulates Chemosensitivity by regulating RAD51 at replication forks. Molecular Cell, 67(3), 374-386.e5. https://doi.org/10.1016/j.molcel.2017.06.023
Eustermann, S., Videler, H., Yang, J.-C., Cole, P. T., Gruszka, D., Veprintsev, D., & Neuhaus, D. (2011). The DNA-binding domain of human PARP-1 interacts with DNA single-strand breaks as a monomer through its second zinc finger. Journal of Molecular Biology, 407(1), 149–170. https://doi.org/10.1016/j.jmb.2011.01.034
Farmer, H., McCabe, N., Lord, C. J., Tutt, A. N. J., Johnson, D. A., Richardson, T. B., Santarosa, M., Dillon, K. J., Hickson, I., Knights, C., Martin, N. M. B., Jackson, S. P., Smith, G. C. M., & Ashworth, A. (2005). Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature, 434(7035), 917–921. https://doi.org/10.1038/nature03445
Ferraris, D. V. (2010). Evolution of poly(ADP-ribose) polymerase-1 (PARP-1) inhibitors. From concept to clinic. Journal of Medicinal Chemistry, 53(12), 4561–4584. https://doi.org/10.1021/jm100012m
Gagné, J.-P., Hunter, J. M., Labrecque, B., Chabot, B., & Poirier, G. G. (2003). A proteomic approach to the identification of heterogeneous nuclear ribonucleoproteins as a new family of poly(ADP-ribose)-binding proteins. The Biochemical Journal, 371(Pt 2), 331–340. https://doi.org/10.1042/BJ20021675
Gagné, J.-P., Isabelle, M., Lo, K. S., Bourassa, S., Hendzel, M. J., Dawson, V. L., Dawson, T. M., & Poirier, G. G. (2008). Proteome-wide identification of poly(ADP-ribose) binding proteins and poly(ADP-ribose)-associated protein complexes. Nucleic Acids Research, 36(22), 6959–6976. https://doi.org/10.1093/nar/gkn771
Ganesan, S. (2018). Tumor suppressor tolerance: Reversion mutations in BRCA1 and BRCA2 and resistance to PARP inhibitors and platinum. JCO Precision Oncology, 2, 1–4. https://doi.org/10.1200/PO.18.00001
Gelmon, K. A., Tischkowitz, M., Mackay, H., Swenerton, K., Robidoux, A., Tonkin, K., Hirte, H., Huntsman, D., Clemons, M., Gilks, B., Yerushalmi, R., Macpherson, E., Carmichael, J., & Oza, A. (2011). Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: A phase 2, multicentre, open-label, non-randomised study. The Lancet Oncology, 12(9), 852–861. https://doi.org/10.1016/S1470-2045(11)70214-5
Gibbs-Seymour, I., Fontana, P., Rack, J. G. M., & Ahel, I. (2016). HPF1/C4orf27 Is a PARP-1-interacting protein that regulates PARP-1 ADP-ribosylation activity. Molecular Cell, 62(3), 432–442. https://doi.org/10.1016/j.molcel.2016.03.008
Gibson, B. A., & Kraus, W. L. (2012). New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nature Reviews Molecular Cell Biology, 13(7), 411–424. https://doi.org/10.1038/nrm3376
Gogola, E., Duarte, A. A., de Ruiter, J. R., Wiegant, W. W., Schmid, J. A., de Bruijn, R., James, D. I., Guerrero Llobet, S., Vis, D. J., Annunziato, S., van den Broek, B., Barazas, M., Kersbergen, A., van de Ven, M., Tarsounas, M., Ogilvie, D. J., van Vugt, M., Wessels, L. F. A., Bartkova, J., & Rottenberg, S. (2018). Selective loss of PARG restores PARylation and counteracts PARP inhibitor-mediated synthetic lethality. Cancer Cell, 33(6), 1078-1093.e12. https://doi.org/10.1016/j.ccell.2018.05.008
Golan, T., Hammel, P., Reni, M., Van Cutsem, E., Macarulla, T., Hall, M. J., Park, J.-O., Hochhauser, D., Arnold, D., Oh, D.-Y., Reinacher-Schick, A., Tortora, G., Algül, H., O’Reilly, E. M., McGuinness, D., Cui, K. Y., Schlienger, K., Locker, G. Y., & Kindler, H. L. (2019). Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. The New England Journal of Medicine, 381(4), 317–327. https://doi.org/10.1056/NEJMoa1903387
González-Martín, A., Pothuri, B., Vergote, I., DePont Christensen, R., Graybill, W., Mirza, M. R., McCormick, C., Lorusso, D., Hoskins, P., Freyer, G., Baumann, K., Jardon, K., Redondo, A., Moore, R. G., Vulsteke, C., O’Cearbhaill, R. E., Lund, B., Backes, F., & Barretina-Ginesta, P. (2019). Niraparib in patients with newly diagnosed advanced ovarian cancer. The New England Journal of Medicine, 381(25), 2391–2402. https://doi.org/10.1056/NEJMoa1910962
Goodall, J., Mateo, J., Yuan, W., Mossop, H., Porta, N., Miranda, S., Perez-Lopez, R., Dolling, D., Robinson, D. R., Sandhu, S., Fowler, G., Ebbs, B., Flohr, P., Seed, G., Rodrigues, D. N., Boysen, G., Bertan, C., Atkin, M., Clarke, M., TOPARP-A investigators. (2017). Circulating cell-free DNA to guide prostate cancer treatment with PARP inhibition. Cancer Discovery, 7(9), 1006–1017. https://doi.org/10.1158/2159-8290.CD-17-0261
Gorodetska, I., Kozeretska, I., & Dubrovska, A. (2019). BRCA genes: The role in genome stability, cancer stemness and therapy resistance. Journal of Cancer, 10(9), 2109–2127. https://doi.org/10.7150/jca.30410
Guillemette, S., Serra, R. W., Peng, M., Hayes, J. A., Konstantinopoulos, P. A., Green, M. R., & Cantor, S. B. (2015). Resistance to therapy in BRCA2 mutant cells due to loss of the nucleosome remodeling factor CHD4. Genes & Development, 29(5), 489–494. https://doi.org/10.1101/gad.256214.114
Haince, J.-F., McDonald, D., Rodrigue, A., Déry, U., Masson, J.-Y., Hendzel, M. J., & Poirier, G. G. (2008). PARP1-dependent kinetics of recruitment of MRE11 and NBS1 proteins to multiple DNA damage sites. The Journal of Biological Chemistry, 283(2), 1197–1208. https://doi.org/10.1074/jbc.M706734200
Hanzlikova, H., Gittens, W., Krejcikova, K., Zeng, Z., & Caldecott, K. W. (2017). Overlapping roles for PARP1 and PARP2 in the recruitment of endogenous XRCC1 and PNKP into oxidized chromatin. Nucleic Acids Research, 45(5), 2546–2557. https://doi.org/10.1093/nar/gkw1246
Hochegger, H., Dejsuphong, D., Fukushima, T., Morrison, C., Sonoda, E., Schreiber, V., Zhao, G. Y., Saberi, A., Masutani, M., Adachi, N., Koyama, H., de Murcia, G., & Takeda, S. (2006). Parp-1 protects homologous recombination from interference by Ku and Ligase IV in vertebrate cells. The EMBO Journal, 25(6), 1305–1314. https://doi.org/10.1038/sj.emboj.7601015
Hu, Q., Botuyan, M. V., Zhao, D., Cui, G., Mer, E., & Mer, G. (2021). Mechanisms of BRCA1–BARD1 nucleosome recognition and ubiquitylation. Nature. https://doi.org/10.1038/s41586-021-03716-8
Huang, D., & Kraus, W. L. (2022). The expanding universe of PARP1-mediated molecular and therapeutic mechanisms. Molecular Cell, 82(12), 2315–2334. https://doi.org/10.1016/j.molcel.2022.02.021
Isabelle, M., Gagné, J.-P., Gallouzi, I.-E., & Poirier, G. G. (2012). Quantitative proteomics and dynamic imaging reveal that G3BP-mediated stress granule assembly is poly(ADP-ribose)-dependent following exposure to MNNG-induced DNA alkylation. Journal of Cell Science, 125(Pt 19), 4555–4566. https://doi.org/10.1242/jcs.106963
Jankevicius, G., Hassler, M., Golia, B., Rybin, V., Zacharias, M., Timinszky, G., & Ladurner, A. G. (2013). A family of macrodomain proteins reverses cellular mono-ADP-ribosylation. Nature Structural & Molecular Biology, 20(4), 508–514. https://doi.org/10.1038/nsmb.2523
Jaspers, J. E., Sol, W., Kersbergen, A., Schlicker, A., Guyader, C., Xu, G., Wessels, L., Borst, P., Jonkers, J., & Rottenberg, S. (2015). BRCA2-deficient sarcomatoid mammary tumors exhibit multidrug resistance. Cancer Research, 75(4), 732–741. https://doi.org/10.1158/0008-5472.CAN-14-0839
Ji, Y., & Tulin, A. V. (2013). Post-transcriptional regulation by poly(ADP-ribosyl)ation of the RNA-binding proteins. International Journal of Molecular Sciences, 14(8), 16168–16183. https://doi.org/10.3390/ijms140816168
Juncheng, P., Joseph, A., Lafarge, A., Martins, I., Obrist, F., Pol, J., Saavedra, E., Li, S., Sauvat, A., Cerrato, G., Lévesque, S., Leduc, M., Kepp, O., Durand, S., Aprahamian, F., Nirmalathansan, N., Michels, J., Kroemer, G., & Castedo, M. (2022). Cancer cell-autonomous overactivation of PARP1 compromises immunosurveillance in non-small cell lung cancer. Journal for Immunotherapy of Cancer, 10(6), e004280. https://doi.org/10.1136/jitc-2021-004280
Kang, H. C., Lee, Y.-I., Shin, J.-H., Andrabi, S. A., Chi, Z., Gagné, J.-P., Lee, Y., Ko, H. S., Lee, B. D., Poirier, G. G., Dawson, V. L., & Dawson, T. M. (2011). Iduna is a poly(ADP-ribose) (PAR)-dependent E3 ubiquitin ligase that regulates DNA damage. Proceedings of the National Academy of Sciences of the United States of America, 108(34), 14103–14108. https://doi.org/10.1073/pnas.1108799108
Kashima, L., Idogawa, M., Mita, H., Shitashige, M., Yamada, T., Ogi, K., Suzuki, H., Toyota, M., Ariga, H., Sasaki, Y., & Tokino, T. (2012). CHFR protein regulates mitotic checkpoint by targeting PARP-1 protein for ubiquitination and degradation. The Journal of Biological Chemistry, 287(16), 12975–12984. https://doi.org/10.1074/jbc.M111.321828
Kassner, I., Barandun, M., Fey, M., Rosenthal, F., & Hottiger, M. O. (2013). Crosstalk between SET7/9-dependent methylation and ARTD1-mediated ADP-ribosylation of histone H1.4. Epigenetics & Chromatin, 6(1), 1. https://doi.org/10.1186/1756-8935-6-1
Kondrashova, O., Nguyen, M., Shield-Artin, K., Tinker, A. V., Teng, N. N. H., Harrell, M. I., Kuiper, M. J., Ho, G.-Y., Barker, H., Jasin, M., Prakash, R., Kass, E. M., Sullivan, M. R., Brunette, G. J., Bernstein, K. A., Coleman, R. L., Floquet, A., Friedlander, M., Kichenadasse, G., AOCS Study Group. (2017). Secondary somatic mutations restoring RAD51C and RAD51D associated with acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discovery, 7(9), 984–998. https://doi.org/10.1158/2159-8290.CD-17-0419
Konstantinopoulos, P. A., Waggoner, S., Vidal, G. A., Mita, M., Moroney, J. W., Holloway, R., Van Le, L., Sachdev, J. C., Chapman-Davis, E., Colon-Otero, G., Penson, R. T., Matulonis, U. A., Kim, Y. B., Moore, K. N., Swisher, E. M., Färkkilä, A., D’Andrea, A., Stringer-Reasor, E., Wang, J., & Munster, P. (2019). Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncology, 5(8), 1141–1149. https://doi.org/10.1001/jamaoncol.2019.1048
Krishnakumar, R., Gamble, M. J., Frizzell, K. M., Berrocal, J. G., Kininis, M., & Kraus, W. L. (2008). Reciprocal binding of PARP-1 and histone H1 at promoters specifies transcriptional outcomes. Science, 319(5864), 819–821. https://doi.org/10.1126/science.1149250
Kristeleit, R., Shapiro, G. I., Burris, H. A., Oza, A. M., LoRusso, P., Patel, M. R., Domchek, S. M., Balmaña, J., Drew, Y., Chen, L.-M., Safra, T., Montes, A., Giordano, H., Maloney, L., Goble, S., Isaacson, J., Xiao, J., Borrow, J., Rolfe, L., & Shapira-Frommer, R. (2017). A phase I-II study of the oral PARP inhibitor rucaparib in patients with germline BRCA1/2-mutated ovarian carcinoma or other solid tumors. Clinical Cancer Research, 23(15), 4095–4106. https://doi.org/10.1158/1078-0432.CCR-16-2796
Langelier, M.-F., Planck, J. L., Roy, S., & Pascal, J. M. (2011). Crystal structures of poly(ADP-ribose) polymerase-1 (PARP-1) zinc fingers bound to DNA: Structural and functional insights into DNA-dependent PARP-1 activity. The Journal of Biological Chemistry, 286(12), 10690–10701. https://doi.org/10.1074/jbc.M110.202507
Langelier, M.-F., Planck, J. L., Roy, S., & Pascal, J. M. (2012). Structural basis for DNA damage-dependent poly(ADP-ribosyl)ation by human PARP-1. Science, 336(6082), 728–732. https://doi.org/10.1126/science.1216338
Langelier, M.-F., Ruhl, D. D., Planck, J. L., Kraus, W. L., & Pascal, J. M. (2010). The Zn3 domain of human poly(ADP-ribose) polymerase-1 (PARP-1) functions in both DNA-dependent poly(ADP-ribose) synthesis activity and chromatin compaction. The Journal of Biological Chemistry, 285(24), 18877–18887. https://doi.org/10.1074/jbc.M110.105668
Ledermann, J., Harter, P., Gourley, C., Friedlander, M., Vergote, I., Rustin, G., Scott, C. L., Meier, W., Shapira-Frommer, R., Safra, T., Matei, D., Fielding, A., Spencer, S., Dougherty, B., Orr, M., Hodgson, D., Barrett, J. C., & Matulonis, U. (2014). Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. The Lancet Oncology, 15(8), 852–861. https://doi.org/10.1016/S1470-2045(14)70228-1
Lepeltier, E., Rijo, P., Rizzolio, F., Popovtzer, R., Petrikaite, V., Assaraf, Y. G., & Passirani, C. (2020). Nanomedicine to target multidrug resistant tumors. Drug Resistance Updates, 52, 100704. https://doi.org/10.1016/j.drup.2020.100704
Leung, A. K. L., Vyas, S., Rood, J. E., Bhutkar, A., Sharp, P. A., & Chang, P. (2011). Poly(ADP-ribose) regulates stress responses and microRNA activity in the cytoplasm. Molecular Cell, 42(4), 489–499. https://doi.org/10.1016/j.molcel.2011.04.015
Li, N., Zhang, Y., Han, X., Liang, K., Wang, J., Feng, L., Wang, W., Songyang, Z., Lin, C., Yang, L., Yu, Y., & Chen, J. (2015). Poly-ADP ribosylation of PTEN by tankyrases promotes PTEN degradation and tumor growth. Genes & Development, 29(2), 157–170. https://doi.org/10.1101/gad.251785.114
Liptay, M., Barbosa, J. S., & Rottenberg, S. (2020). Replication fork remodeling and therapy escape in DNA damage response-deficient cancers. Frontiers in Oncology, 10, 670. https://doi.org/10.3389/fonc.2020.00670
Litton, J. K., Rugo, H. S., Ettl, J., Hurvitz, S. A., Gonçalves, A., Lee, K.-H., Fehrenbacher, L., Yerushalmi, R., Mina, L. A., Martin, M., Roché, H., Im, Y.-H., Quek, R. G. W., Markova, D., Tudor, I. C., Hannah, A. L., Eiermann, W., & Blum, J. L. (2018). Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. The New England Journal of Medicine, 379(8), 753–763. https://doi.org/10.1056/NEJMoa1802905
Liu, J. F., Barry, W. T., Birrer, M., Lee, J.-M., Buckanovich, R. J., Fleming, G. F., Rimel, B., Buss, M. K., Nattam, S., Hurteau, J., Luo, W., Quy, P., Whalen, C., Obermayer, L., Lee, H., Winer, E. P., Kohn, E. C., Ivy, S. P., & Matulonis, U. A. (2014). Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: A randomised phase 2 study. The Lancet Oncology, 15(11), 1207–1214. https://doi.org/10.1016/S1470-2045(14)70391-2
Loehr, A., Hussain, A., Patnaik, A., Bryce, A. H., Castellano, D., Font, A., Shapiro, J., Zhang, J., Sautois, B., Vogelzang, N. J., Chatta, G., Courtney, K., Harzstark, A., Ricci, F., Despain, D., Watkins, S., King, C., Nguyen, M., Simmons, A. D., & Abida, W. (2022). Emergence of BRCA reversion mutations in patients with metastatic castration-resistant prostate cancer after treatment with rucaparib. European Urology, S0302–2838(22), 02639–02642. https://doi.org/10.1016/j.eururo.2022.09.010
Lombard, A. P., Liu, C., Armstrong, C. M., D’Abronzo, L. S., Lou, W., Chen, H., Dall’Era, M., Ghosh, P. M., Evans, C. P., & Gao, A. C. (2019). Overexpressed ABCB1 induces olaparib-taxane cross-resistance in advanced prostate cancer. Translational Oncology, 12(7), 871–878. https://doi.org/10.1016/j.tranon.2019.04.007
Lord, C. J., & Ashworth, A. (2017). PARP inhibitors: Synthetic lethality in the clinic. Science, 355(6330), 1152–1158. https://doi.org/10.1126/science.aam7344
Mallery, D. L., Vandenberg, C. J., & Hiom, K. (2002). Activation of the E3 ligase function of the BRCA1/BARD1 complex by polyubiquitin chains. The EMBO Journal, 21(24), 6755–6762. https://doi.org/10.1093/emboj/cdf691
Manco, G., Lacerra, G., Porzio, E., & Catara, G. (2022). ADP-ribosylation post-translational modification: An overview with a focus on RNA biology and new pharmacological perspectives. Biomolecules, 12(3), 443. https://doi.org/10.3390/biom12030443
Manke, I. A., Lowery, D. M., Nguyen, A., & Yaffe, M. B. (2003). BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science, 302(5645), 636–639. https://doi.org/10.1126/science.1088877
Maruta, H., Okita, N., Takasawa, R., Uchiumi, F., Hatano, T., & Tanuma, S. (2007). The involvement of ATP produced via (ADP-Ribose)n in the maintenance of DNA replication apparatus during DNA repair. Biological & Pharmaceutical Bulletin, 30(3), 447–450. https://doi.org/10.1248/bpb.30.447
Mashimo, M., Onishi, M., Uno, A., Tanimichi, A., Nobeyama, A., Mori, M., Yamada, S., Negi, S., Bu, X., Kato, J., Moss, J., Sanada, N., Kizu, R., & Fujii, T. (2021). The 89-kDa PARP1 cleavage fragment serves as a cytoplasmic PAR carrier to induce AIF-mediated apoptosis. Journal of Biological Chemistry. https://doi.org/10.1074/jbc.RA120.014479
Matveeva, E., Maiorano, J., Zhang, Q., Eteleeb, A. M., Convertini, P., Chen, J., Infantino, V., Stamm, S., Wang, J., Rouchka, E. C., & Fondufe-Mittendorf, Y. N. (2016). Involvement of PARP1 in the regulation of alternative splicing. Cell Discovery, 2, 15046. https://doi.org/10.1038/celldisc.2015.46
Messner, S., Altmeyer, M., Zhao, H., Pozivil, A., Roschitzki, B., Gehrig, P., Rutishauser, D., Huang, D., Caflisch, A., & Hottiger, M. O. (2010). PARP1 ADP-ribosylates lysine residues of the core histone tails. Nucleic Acids Research, 38(19), 6350–6362. https://doi.org/10.1093/nar/gkq463
Mirza, M. R., Åvall Lundqvist, E., Birrer, M. J., dePont Christensen, R., Nyvang, G.-B., Malander, S., Anttila, M., Werner, T. L., Lund, B., Lindahl, G., Hietanen, S., Peen, U., Dimoula, M., Roed, H., Ør Knudsen, A., Staff, S., Krog Vistisen, A., Bjørge, L., Mäenpää, J. U., AVANOVA investigators. (2019). Niraparib plus bevacizumab versus niraparib alone for platinum-sensitive recurrent ovarian cancer (NSGO-AVANOVA2/ENGOT-ov24): A randomised, phase 2, superiority trial. The Lancet. Oncology, 20(10), 1409–1419. https://doi.org/10.1016/S1470-2045(19)30515-7
Mirza, M. R., Monk, B. J., Herrstedt, J., Oza, A. M., Mahner, S., Redondo, A., Fabbro, M., Ledermann, J. A., Lorusso, D., Vergote, I., Ben-Baruch, N. E., Marth, C., Mądry, R., Christensen, R. D., Berek, J. S., Dørum, A., Tinker, A. V., du Bois, A., González-Martín, A., ENGOT-OV16/NOVA Investigators. (2016). Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. The New England Journal of Medicine, 375(22), 2154–2164. https://doi.org/10.1056/NEJMoa1611310
Moore, K. N., Secord, A. A., Geller, M. A., Miller, D. S., Cloven, N., Fleming, G. F., Wahner Hendrickson, A. E., Azodi, M., DiSilvestro, P., Oza, A. M., Cristea, M., Berek, J. S., Chan, J. K., Rimel, B. J., Matei, D. E., Li, Y., Sun, K., Luptakova, K., Matulonis, U. A., & Monk, B. J. (2019). Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): A multicentre, open-label, single-arm, phase 2 trial. The Lancet Oncology, 20(5), 636–648. https://doi.org/10.1016/S1470-2045(19)30029-4
Moudry, P., Watanabe, K., Wolanin, K. M., Bartkova, J., Wassing, I. E., Watanabe, S., Strauss, R., Troelsgaard Pedersen, R., Oestergaard, V. H., Lisby, M., Andújar-Sánchez, M., Maya-Mendoza, A., Esashi, F., Lukas, J., & Bartek, J. (2016). TOPBP1 regulates RAD51 phosphorylation and chromatin loading and determines PARP inhibitor sensitivity. The Journal of Cell Biology, 212(3), 281–288. https://doi.org/10.1083/jcb.201507042
Mukhopadhyay, A., Elattar, A., Cerbinskaite, A., Wilkinson, S. J., Drew, Y., Kyle, S., Los, G., Hostomsky, Z., Edmondson, R. J., & Curtin, N. J. (2010). Development of a functional assay for homologous recombination status in primary cultures of epithelial ovarian tumor and correlation with sensitivity to poly(ADP-ribose) polymerase inhibitors. Clinical Cancer Research, 16(8), 2344–2351. https://doi.org/10.1158/1078-0432.CCR-09-2758
Murai, J., Huang, S.-Y.N., Renaud, A., Zhang, Y., Ji, J., Takeda, S., Morris, J., Teicher, B., Doroshow, J. H., & Pommier, Y. (2014). Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Molecular Cancer Therapeutics, 13(2), 433–443. https://doi.org/10.1158/1535-7163.MCT-13-0803
Niere, M., Mashimo, M., Agledal, L., Dölle, C., Kasamatsu, A., Kato, J., Moss, J., & Ziegler, M. (2012). ADP-ribosylhydrolase 3 (ARH3), not poly(ADP-ribose) glycohydrolase (PARG) isoforms, Is responsible for degradation of mitochondrial matrix-associated poly(ADP-ribose)*. Journal of Biological Chemistry, 287(20), 16088–16102. https://doi.org/10.1074/jbc.M112.349183
Norquist, B., Wurz, K. A., Pennil, C. C., Garcia, R., Gross, J., Sakai, W., Karlan, B. Y., Taniguchi, T., & Swisher, E. M. (2011). Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. Journal of Clinical Oncology, 29(22), 3008–3015. https://doi.org/10.1200/JCO.2010.34.2980
Oka, S., Kato, J., & Moss, J. (2006). Identification and characterization of a mammalian 39-kDa poly(ADP-ribose) glycohydrolase. The Journal of Biological Chemistry, 281(2), 705–713. https://doi.org/10.1074/jbc.M510290200
Palazzo, L., Leidecker, O., Prokhorova, E., Dauben, H., Matic, I., & Ahel, I. (2018). Serine is the major residue for ADP-ribosylation upon DNA damage. eLife, 7, e34334. https://doi.org/10.7554/eLife.34334
Park, H., Kam, T.-I., Dawson, T. M., & Dawson, V. L. (2020). Poly (ADP-ribose) (PAR)-dependent cell death in neurodegenerative diseases. International Review of Cell and Molecular Biology, 353, 1–29. https://doi.org/10.1016/bs.ircmb.2019.12.009
Patch, A.-M., Christie, E. L., Etemadmoghadam, D., Garsed, D. W., George, J., Fereday, S., Nones, K., Cowin, P., Alsop, K., Bailey, P. J., Kassahn, K. S., Newell, F., Quinn, M. C. J., Kazakoff, S., Quek, K., Wilhelm-Benartzi, C., Curry, E., Leong, H. S., Australian Ovarian Cancer Study Group, & Bowtell, D. D. L. (2015). Whole-genome characterization of chemoresistant ovarian cancer. Nature, 521(7553), 489–494. https://doi.org/10.1038/nature14410
Patel, A. G., Sarkaria, J. N., & Kaufmann, S. H. (2011). Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proceedings of the National Academy of Sciences of the United States of America, 108(8), 3406–3411. https://doi.org/10.1073/pnas.1013715108
Peterson, F. C., Chen, D., Lytle, B. L., Rossi, M. N., Ahel, I., Denu, J. M., & Volkman, B. F. (2011). Orphan macrodomain protein (human C6orf130) is an O-acyl-ADP-ribose deacylase: Solution structure and catalytic properties. The Journal of Biological Chemistry, 286(41), 35955–35965. https://doi.org/10.1074/jbc.M111.276238
Pillay, N., Tighe, A., Nelson, L., Littler, S., Coulson-Gilmer, C., Bah, N., Golder, A., Bakker, B., Spierings, D. C. J., James, D. I., Smith, K. M., Jordan, A. M., Morgan, R. D., Ogilvie, D. J., Foijer, F., Jackson, D. A., & Taylor, S. S. (2019). DNA replication vulnerabilities render ovarian cancer cells sensitive to poly(ADP-Ribose) glycohydrolase inhibitors. Cancer Cell, 35(3), 519-533.e8. https://doi.org/10.1016/j.ccell.2019.02.004
Plummer, R., Jones, C., Middleton, M., Wilson, R., Evans, J., Olsen, A., Curtin, N., Boddy, A., McHugh, P., Newell, D., Harris, A., Johnson, P., Steinfeldt, H., Dewji, R., Wang, D., Robson, L., & Calvert, H. (2008). Phase I study of the poly(ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors. Clinical Cancer Research, 14(23), 7917–7923. https://doi.org/10.1158/1078-0432.CCR-08-1223
Poveda, A., Floquet, A., Ledermann, J. A., Asher, R., Penson, R. T., Oza, A. M., Korach, J., Huzarski, T., Pignata, S., Friedlander, M., Baldoni, A., Park-Simon, T.-W., Tamura, K., Sonke, G. S., Lisyanskaya, A., Kim, J.-H., Filho, E. A., Milenkova, T., Lowe, E. S., SOLO2/ENGOT-Ov21 investigators. (2021). Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. The Lancet Oncology, 22(5), 620–631. https://doi.org/10.1016/S1470-2045(21)00073-5
Powell, C., Mikropoulos, C., Kaye, S. B., Nutting, C. M., Bhide, S. A., Newbold, K., & Harrington, K. J. (2010). Pre-clinical and clinical evaluation of PARP inhibitors as tumour-specific radiosensitisers. Cancer Treatment Reviews, 36(7), 566–575. https://doi.org/10.1016/j.ctrv.2010.03.003
Prokhorova, E., Agnew, T., Wondisford, A. R., Tellier, M., Kaminski, N., Beijer, D., Holder, J., Groslambert, J., Suskiewicz, M. J., Zhu, K., Reber, J. M., Krassnig, S. C., Palazzo, L., Murphy, S., Nielsen, M. L., Mangerich, A., Ahel, D., Baets, J., O’Sullivan, R. J., & Ahel, I. (2021). Unrestrained poly-ADP-ribosylation provides insights into chromatin regulation and human disease. Molecular Cell, 81(12), 2640-2655.e8. https://doi.org/10.1016/j.molcel.2021.04.028
Rack, J. G. M., Perina, D., & Ahel, I. (2016). Macrodomains: structure, function, evolution, and catalytic activities. Annual Review of Biochemistry, 85, 431–454. https://doi.org/10.1146/annurev-biochem-060815-014935
Rafiei, S., Fitzpatrick, K., Liu, D., Cai, M.-Y., Elmarakeby, H. A., Park, J., Ricker, C., Kochupurakkal, B. S., Choudhury, A. D., Hahn, W. C., Balk, S. P., Hwang, J. H., Van Allen, E. M., & Mouw, K. W. (2020). ATM loss confers greater sensitivity to ATR inhibition than PARP inhibition in prostate cancer. Cancer Research, 80(11), 2094–2100. https://doi.org/10.1158/0008-5472.CAN-19-3126
Raphael, B. J., Hruban, R. H., Aguirre, A. J., Moffitt, R. A., Yeh, J. J., Stewart, C., Robertson, A. G., Cherniack, A. D., Gupta, M., Getz, G., Gabriel, S. B., Meyerson, M., Cibulskis, C., Fei, S. S., Hinoue, T., Shen, H., Laird, P. W., Ling, S., Lu, Y., & Zenklusen, J. C. (2017). Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell, 32(2), 185-203.e13. https://doi.org/10.1016/j.ccell.2017.07.007
Ray Chaudhuri, A., Callen, E., Ding, X., Gogola, E., Duarte, A. A., Lee, J.-E., Wong, N., Lafarga, V., Calvo, J. A., Panzarino, N. J., John, S., Day, A., Crespo, A. V., Shen, B., Starnes, L. M., de Ruiter, J. R., Daniel, J. A., Konstantinopoulos, P. A., Cortez, D., & Nussenzweig, A. (2016). Replication fork stability confers chemoresistance in BRCA-deficient cells. Nature, 535(7612), 382–387. https://doi.org/10.1038/nature18325
Ray Chaudhuri, A., & Nussenzweig, A. (2017). The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nature Reviews Molecular Cell Biology, 18(10), 610–621. https://doi.org/10.1038/nrm.2017.53
Ray-Coquard, I., Pautier, P., Pignata, S., Pérol, D., González-Martín, A., Berger, R., Fujiwara, K., Vergote, I., Colombo, N., Mäenpää, J., Selle, F., Sehouli, J., Lorusso, D., Guerra Alía, E. M., Reinthaller, A., Nagao, S., Lefeuvre-Plesse, C., Canzler, U., Scambia, G., PAOLA-1 Investigators. (2019). Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. The New England Journal of Medicine, 381(25), 2416–2428. https://doi.org/10.1056/NEJMoa1911361
Rebbeck, T. R., Mitra, N., Wan, F., Sinilnikova, O. M., Healey, S., McGuffog, L., Mazoyer, S., Chenevix-Trench, G., Easton, D. F., Antoniou, A. C., Nathanson, K. L., CIMBA Consortium, Laitman, Y., Kushnir, A., Paluch-Shimon, S., Berger, R., Zidan, J., Friedman, E., Ehrencrona, H., & Andrulis, I. (2015). Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA, 313(13), 1347–1361. https://doi.org/10.1001/jama.2014.5985
Robinson, D., Van Allen, E. M., Wu, Y.-M., Schultz, N., Lonigro, R. J., Mosquera, J.-M., Montgomery, B., Taplin, M.-E., Pritchard, C. C., Attard, G., Beltran, H., Abida, W., Bradley, R. K., Vinson, J., Cao, X., Vats, P., Kunju, L. P., Hussain, M., Feng, F. Y., & Chinnaiyan, A. M. (2015). Integrative clinical genomics of advanced prostate cancer. Cell, 161(5), 1215–1228. https://doi.org/10.1016/j.cell.2015.05.001
Robson, M., Im, S.-A., Senkus, E., Xu, B., Domchek, S. M., Masuda, N., Delaloge, S., Li, W., Tung, N., Armstrong, A., Wu, W., Goessl, C., Runswick, S., & Conte, P. (2017). Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. The New England Journal of Medicine, 377(6), 523–533. https://doi.org/10.1056/NEJMoa1706450
Rolli, V., O’Farrell, M., Ménissier-de Murcia, J., & de Murcia, G. (1997). Random mutagenesis of the poly(ADP-ribose) polymerase catalytic domain reveals amino acids involved in polymer branching. Biochemistry, 36(40), 12147–12154. https://doi.org/10.1021/bi971055p
Rondinelli, B., Gogola, E., Yücel, H., Duarte, A. A., van de Ven, M., van der Sluijs, R., Konstantinopoulos, P. A., Jonkers, J., Ceccaldi, R., Rottenberg, S., & D’Andrea, A. D. (2017). EZH2 promotes degradation of stalled replication forks by recruiting MUS81 through histone H3 trimethylation. Nature Cell Biology, 19(11), 1371–1378. https://doi.org/10.1038/ncb3626
Rosenthal, F., Feijs, K. L. H., Frugier, E., Bonalli, M., Forst, A. H., Imhof, R., Winkler, H. C., Fischer, D., Caflisch, A., Hassa, P. O., Lüscher, B., & Hottiger, M. O. (2013). Macrodomain-containing proteins are new mono-ADP-ribosylhydrolases. Nature Structural & Molecular Biology, 20(4), 502–507. https://doi.org/10.1038/nsmb.2521
Ruf, A., Mennissier de Murcia, J., de Murcia, G., & Schulz, G. E. (1996). Structure of the catalytic fragment of poly(AD-ribose) polymerase from chicken. Proceedings of the National Academy of Sciences of the United States of America, 93(15), 7481–7485. https://doi.org/10.1073/pnas.93.15.7481
Ruf, A., Rolli, V., de Murcia, G., & Schulz, G. E. (1998). The mechanism of the elongation and branching reaction of poly(ADP-ribose) polymerase as derived from crystal structures and mutagenesis. Journal of Molecular Biology, 278(1), 57–65. https://doi.org/10.1006/jmbi.1998.1673
Schlacher, K., Christ, N., Siaud, N., Egashira, A., Wu, H., & Jasin, M. (2011). Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell, 145(4), 529–542. https://doi.org/10.1016/j.cell.2011.03.041
Sharifi, R., Morra, R., Appel, C. D., Tallis, M., Chioza, B., Jankevicius, G., Simpson, M. A., Matic, I., Ozkan, E., Golia, B., Schellenberg, M. J., Weston, R., Williams, J. G., Rossi, M. N., Galehdari, H., Krahn, J., Wan, A., Trembath, R. C., Crosby, A. H., & Ahel, I. (2013). Deficiency of terminal ADP-ribose protein glycohydrolase TARG1/C6orf130 in neurodegenerative disease. The EMBO Journal, 32(9), 1225–1237. https://doi.org/10.1038/emboj.2013.51
Slade, D., Dunstan, M. S., Barkauskaite, E., Weston, R., Lafite, P., Dixon, N., Ahel, M., Leys, D., & Ahel, I. (2011). The structure and catalytic mechanism of a poly(ADP-ribose) glycohydrolase. Nature, 477(7366), 616–620. https://doi.org/10.1038/nature10404
Smith, R., Lebeaupin, T., Juhász, S., Chapuis, C., D’Augustin, O., Dutertre, S., Burkovics, P., Biertümpfel, C., Timinszky, G., & Huet, S. (2019). Poly(ADP-ribose)-dependent chromatin unfolding facilitates the association of DNA-binding proteins with DNA at sites of damage. Nucleic Acids Research, 47(21), 11250–11267. https://doi.org/10.1093/nar/gkz820
Solmaz, A. E., Onay, H., Yeniay, L., Gökmen, E., Özdemir, N., Alanyalı, S., Oktay, A., Özsaran, Z., Kapkaç, M., & Özkınay, F. (2020). BRCA1-BRCA2 mutation analysis results in 910 individuals: Mutation distribution and 8 novel mutations. Cancer Genetics, 241, 20–24. https://doi.org/10.1016/j.cancergen.2019.12.008
Stewart, R. A., Pilié, P. G., & Yap, T. A. (2018). Development of PARP and Immune-checkpoint inhibitor combinations. Cancer Research, 78(24), 6717–6725. https://doi.org/10.1158/0008-5472.CAN-18-2652
Vyas, S., Chesarone-Cataldo, M., Todorova, T., Huang, Y.-H., & Chang, P. (2013). A systematic analysis of the PARP protein family identifies new functions critical for cell physiology. Nature Communications, 4, 2240. https://doi.org/10.1038/ncomms3240
Vyas, S., Matic, I., Uchima, L., Rood, J., Zaja, R., Hay, R. T., Ahel, I., & Chang, P. (2014). Family-wide analysis of poly(ADP-ribose) polymerase activity. Nature Communications, 5, 4426. https://doi.org/10.1038/ncomms5426
Waks, A. G., Cohen, O., Kochupurakkal, B., Kim, D., Dunn, C. E., Buendia Buendia, J., Wander, S., Helvie, K., Lloyd, M. R., Marini, L., Hughes, M. E., Freeman, S. S., Ivy, S. P., Geradts, J., Isakoff, S., LoRusso, P., Adalsteinsson, V. A., Tolaney, S. M., Matulonis, U., & Wagle, N. (2020). Reversion and non-reversion mechanisms of resistance to PARP inhibitor or platinum chemotherapy in BRCA1/2-mutant metastatic breast cancer. Annals of Oncology, 31(5), 590–598. https://doi.org/10.1016/j.annonc.2020.02.008
Whitehouse, C. J., Taylor, R. M., Thistlethwaite, A., Zhang, H., Karimi-Busheri, F., Lasko, D. D., Weinfeld, M., & Caldecott, K. W. (2001). XRCC1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair. Cell, 104(1), 107–117. https://doi.org/10.1016/s0092-8674(01)00195-7
Xu, Y., Huang, S., Liu, Z.-G., & Han, J. (2006). Poly(ADP-ribose) polymerase-1 signaling to mitochondria in necrotic cell death requires RIP1/TRAF2-mediated JNK1 activation. The Journal of Biological Chemistry, 281(13), 8788–8795. https://doi.org/10.1074/jbc.M508135200
Yang, L., Zhang, Y., Shan, W., Hu, Z., Yuan, J., Pi, J., Wang, Y., Fan, L., Tang, Z., Li, C., Hu, X., Tanyi, J. L., Fan, Y., Huang, Q., Montone, K., Dang, C. V., & Zhang, L. (2017). Repression of BET activity sensitizes homologous recombination-proficient cancers to PARP inhibition. Science Translational Medicine, 9(400), eaal1645. https://doi.org/10.1126/scitranslmed.aal1645
Yu, J., Hu, X., Yang, Q., Shan, R., Zhang, Y., Dong, Z., Li, H., Wang, J., Li, C., Xie, S., Dong, Y., Ni, W., Jiang, L., Liu, X., Wei, B., Wen, J., Liu, M., Chen, Q., Yang, Y., & Meng, X. (2022). Insulin-like growth factor binding protein 7 promotes acute kidney injury by alleviating poly ADP ribose polymerase 1 degradation. Kidney International, 102(4), 828–844. https://doi.org/10.1016/j.kint.2022.05.026
Yu, S.-W., Andrabi, S. A., Wang, H., Kim, N. S., Poirier, G. G., Dawson, T. M., & Dawson, V. L. (2006). Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proceedings of the National Academy of Sciences of the United States of America, 103(48), 18314–18319. https://doi.org/10.1073/pnas.0606528103
Yu, S.-W., Wang, H., Poitras, M. F., Coombs, C., Bowers, W. J., Federoff, H. J., Poirier, G. G., Dawson, T. M., & Dawson, V. L. (2002). Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science, 297(5579), 259–263. https://doi.org/10.1126/science.1072221
Zhang, Y., Liu, S., Mickanin, C., Feng, Y., Charlat, O., Michaud, G. A., Schirle, M., Shi, X., Hild, M., Bauer, A., Myer, V. E., Finan, P. M., Porter, J. A., Huang, S.-M.A., & Cong, F. (2011). RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin degradation and Wnt signalling. Nature Cell Biology, 13(5), 623–629. https://doi.org/10.1038/ncb2222
Zhou, B., Yan, J., Guo, L., Zhang, B., Liu, S., Yu, M., Chen, Z., Zhang, K., Zhang, W., Li, X., Xu, Y., Xiao, Y., Zhou, J., Fan, J., Hung, M.-C., Li, H., & Ye, Q. (2020). Hepatoma cell-intrinsic TLR9 activation induces immune escape through PD-L1 upregulation in hepatocellular carcinoma. Theranostics, 10(14), 6530–6543. https://doi.org/10.7150/thno.44417
Acknowledgements
This work was supported by National Natural Science Foundation of China (T2225006) and Beijing Municipal Natural Science Foundation (Z220011) to ML.
Author information
Authors and Affiliations
Contributions
ML designed and wrote the manuscript. YG wrote the manuscript. BF revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, Y., Fan, B. & Li, M. PARP molecular functions and applications of PARP inhibitors in cancer treatment. GENOME INSTAB. DIS. 4, 137–153 (2023). https://doi.org/10.1007/s42764-023-00100-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42764-023-00100-w