Abstract
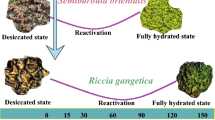
To better understand the mechanism of desiccation tolerance of resurrection fern allies Selaginella wightii Hieron and Selaginella involvens (S.W.) Spring, the present study was planned to compare the quantitative profile of photosynthetic pigments, carotenoids, amino acids, proline, protein, IAA and non reducing sugar content of desiccated, rehydrated and fresh samples of Selaginella wightii Hieron and Selaginella involvens (S.W.) Spring. The variation of photosynthetic pigments, amino acids, proline, protein, indole acetic acid (IAA) and reducing sugar content of desiccated and rehydrated samples of S. wightii and S. involvens were estimated using the standard procedure. The ten months dehydrated samples of S. wightii and S. involvens showed high concentration of chlorophyll, carotenoids, proteins, amino acids, proline and IAA. The observed biochemical results confirm the resurrection and desiccation tolerant potential of Selaginella wightii Hieron and Selaginella involvens (S.W.) Spring. The observed chlorophyll results suggested that the studied two fern allies are homoiochlorophyllous plants. The reducing sugar content was high in fresh samples and low amount in the ten months dehydrated samples of S. wightii and S. involvens. It may be due to the conversion of reducing sugar to non-reducing sugar to tolerate the desiccation. The biochemical composition change confirms the resurrection potential of S. wightii and S. involvens.





Similar content being viewed by others
Abbreviations
- IAA:
-
Indole acetic acid
- XCH:
-
St. Xavier’s college herbarium
- TMD:
-
Ten months dehydrated
- DW:
-
Dry weight
- FW:
-
Fresh weight
References
Adnan M, Siddiqui AJ, Hamadou WS, Snoussi M, Badraoui R, Ashraf SA, Jamal A, Awadelkareem AM, Sachidanandan M, Hadi S et al (2021a) Deciphering the molecular mechanism responsible for efficiently inhibiting metastasis of human non-small cell lung and colorectal cancer cells targeting the matrix metalloproteinases by Selaginella repanda. Plants 10:979. https://doi.org/10.3390/plants10050979
Adnan M, Siddiqui AJ, Jamal A, Hamadou WS, Awadelkareem AM, Manojkumar S, Patel M (2021b) Evidence-based medicinal potential and possible role of selaginella in the prevention of modern chronic diseases: ethnopharmacological and ethnobotanical perspective. Rec Nat Prod 15(5):330–355
Adnan M, Siddiqui AJ, Hamadou WS, Patel M, Ashraf SA, Jamal A, Awadelkareem AM, Sachidanandan M, Snoussi M, De Feo V (2021c) Phytochemistry, bioactivities, pharmacokinetics and toxicity prediction of Selaginella repanda with its anticancer potential against human lung, breast and colorectal carcinoma cell lines. Molecules 26:768. https://doi.org/10.3390/molecules26030768
Akram NA, Shafiq F, Ashraf M (2017) Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front Plant Sci 8:613. https://doi.org/10.3389/fpls.2017.00613
Anjum SA, Xie X, Wang L, Saleem FM, Man C, Lei W (2011) Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agr 6:2026–2032. https://doi.org/10.5897/AJAR10.027
Arnon DI (1949) Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta Vulgaris. Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.1
Berens ML, Wolinska KW, Spaepen S, Ziegler J, Nobori T, Nair A et al (2019) Balancing trade-offs between biotic and abiotic stress responses through leaf age-dependent variation in stress hormone cross-talk. Proc Nat Acad Sci USA 116:2364–2373. https://doi.org/10.1073/pnas.1817233116
Bewley DA (1979) Physiological aspects of desiccation tolerance. Annu Rev Plant Physiol 30:195–238
Bianchi G, Murelli C, Bochicchio A, Vazzana C (1991) Changes in low- molecular weight substances in Boea hygroscopica in response to desiccation and rehydration. Phytochemistry 30(2):461–466. https://doi.org/10.1016/0031-9422(91)83705-P
Bijanzadeh E, Emam Y (2010) Effect of defoliation and drought stress on yield components and chlorophyll content of wheat. Pak J Biol Sci 13:699–705. https://doi.org/10.3923/pjbs.2010.699.705
Cascante M, Marin S (2008) Metabolomics and fluxomics approaches. Essays Biochem 45:67–81. https://doi.org/10.1042/BSE0450067
Chai TT, Wong FC (2012) Antioxidant properties of aqueous extracts of Selaginella willdenowii. J Med Plants Res 6(7):1289–1296. https://doi.org/10.5897/JMPR11.1376
Chen LS, Li P, Cheng L (2009) Comparison of thermotolerance of sun-exposed peel and shaded peel of Fuji apple. Environ Exp Bot 66(1):110–116. https://doi.org/10.1016/j.envexpbot.2008.12.017
Cook D, Fowler S, Fiehn O (2004) A prominent role for the CBF cold response pathway in configuring the low-temperature metabolome of Arabidopsis. Proc Natl Acad Sci USA Crop Sci 50:1037–1047. https://doi.org/10.1073/pnas.0406069101
De SI, Lau S, Voss U (2010) Bimodular auxin response controls organogenesis in Arabidopsis. Proc Natl Acad Sci 107:2705–2710
Din J, Khan SU, Ali I, Gurmani AR (2011) Physiological and agronomic response of canola varieties to drought stress. J Anim Plant Sci 21:78–82
Dinakar C, Bartels D (2012) Light response, oxidative stress management and nucleic acid stability in closely related Linderniaceae species differing in desiccation tolerance. Planta 236:541–555. https://doi.org/10.1007/s00425-012-1628-8
Djilianov DL, Dobrev PI, Moyankova DP, Vankova R, Georgieva DT, Gajdošová S, Motyka V (2013) Dynamics of endogenous phytohormones during desiccation and recovery of the resurrection plant species Haberlea rhodopensis. J Plant Growth Regul 32:564–574
El-Sabagh A, Hossain A, Islam MS, Dinajpur B, Iqbal MA, Aftab T et al (2021a) Prospective role of plant growth regulators for tolerance to abiotic stresses. In: Aftab T, Hakeem KR (eds) Plant growth regulators: signalling under stress conditions. Springer Nature Switzerland AG, Switzerland, pp 1–38
El-Sabagh A, Çig F, Seydosoglu S, Battaglia ML, Javed T, Iqbal MA et al (2021b) Salinity stress in maize: effects of stress and recent developments of tolerance for improvement. Cereal Grains. IntechOpen, London, pp 1–20
Fahad S, Hussain S, Bano A, Saud S, Hassan S, Shan D, Khan FA, Khan F, Chen YT, Wu C, Tabassum MA, Chun MX, Afzal M, Jan A, Jan MT, Huang JL (2015) Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: consequences for changing environment. Environ Sci Pollut. 22:4907–4921. https://doi.org/10.1007/s11356-014-3754-2
Farrant JM, Brandt W, Lindsey GG (2007) An Overview of mechanisms of desiccation tolerance in selected angiosperm resurrection plants. Plant Stress 1(1):72–84
Farrant J, Lehner A, Cooper K, Wiswedel S (2009) Desiccation tolerance in the vegetative tissues of the fern Mohria caffrorum is seasonally regulated. Plant J 57:65–79. https://doi.org/10.1111/j.1365-313X.2008.03673.x
Friedman W (2011) Plant genomics: homoplasy heaven in a lycophyte genome. Curr Biol 21:554–556. https://doi.org/10.1016/j.cub.2011.05.055
Gaff DF (1989) Responses of desiccation tolerant “resurrection” plants to water stress. In: Kreeb KH, Richter H, Hinkley TM (eds) Structural and functional responses to environmental stresses. SPB Academic Publishing, The Hague, pp 255–268
Gaff D, McGregor G (1979) The effect of dehydration and rehydration on the nitrogen content of various fractions from resurrection plants. Biol Plant 1979(21):92–99
Georgieva K, Doncheva S, Mihailova G, Petkova S (2013) Effect of light on the photosynthetic activity during desiccation of the resurrection plant Haberlea rhodopensis. In: Photosynthesis research for food, fuel and the future. Advanced topics in science and technology in China. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-32034-7_113
Gordon SA, Weber RP (1951) Colorimetric estimation of indole acetic acid. Plant Physiol. https://doi.org/10.1104/pp.26.1.192
Greeshma GM, Murugan K (2015) Desiccation tolerance in artillery plant Pilea microphylla L. Liebm. Int Res J Environ Sci 4(12):26–32
Huseynova M, Suleymanov SY, Rustamova SM, Aliyev JA (2009) Drought-induced changes in photosynthetic membranes of two wheat Triticum aestivum L. cultivars. Russ Biokhimiya 74:1109–1116. https://doi.org/10.1134/S0006297909080124
Iqbal N, Umar S, Khan NA, Khan MIR (2014) A new perspective of phytohormones in salinity tolerance: regulation of proline metabolism. Environ Exp Bot 100:34–42. https://doi.org/10.1016/j.envexpbot.2013.12.006
Kalaiarasi V, Johnson M (2018) Docking studies of stress tolerant proteins with protective Molecules. Int J Adv Res Comput Sci 9(1):527–535
Kannan ND, Kulandaivelu G (2011) Drought induced changes in physiological, biochemical and phytochemical properties of Withania somnifera Dun. J Med Plants Res 5:3929–3935
Kaplan F, Guy CL (2004) Beta-amylase induction and the protective role of maltose during temperature shock. Plant Physiol 135:1674–1684. https://doi.org/10.1104/pp.104.040808
Kostal V, Zahradnickova H, Simek P (2011) Hyper prolinemic larvae of the drosophilid fly, Chymomyza costata, survive cryopreservation in liquid nitrogen. Proc Natl Acad Sci USA 108:13041–13046
Ku YS, Sintaha M, Cheung MY, Lam HM (2018) Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int J Mol Sci 19:3206. https://doi.org/10.3390/ijms19103206
Liu GG, Zhijuan W, Hongtao J, Fupeng M (2014) Auxin in plant growth and stress responses. In: Tran LS, Pal S (eds) Phytohormones: a window to metabolism, signaling and biotechnological applications. Springer, New York, NY, pp 1–35
Lowry H, Rosebrough J, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Ma N, Hu C, Wan L, Hu Q, Xiong J, Zhang C (2017) Strigolactones improve plant growth, photosynthesis, and alleviate oxidative stress under salinity in rapeseed (Brassica napus L.) by regulating gene expression. Front Plant Sci 8:1671. https://doi.org/10.3389/fpls.2017.01671
Mafakheri A, Siosemardeh A, Bahramnejad B, Sttruik PC, Sohrabi E (2010) Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust J Crop Sci 4:580–585
Manickam VS, Irudayaraj V (1992) Pteridophyte flora of the Western Ghats, South India. B. I. Publications, New Delhi
Manivannan P, Abdul JC, Sankar B, Kishorekumar A, Somasundaram R, Lakshmanan GMA, Panneerselvam R (2007) Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Colloids Surf, B 59:141–149. https://doi.org/10.1016/j.colsurfb.2007.05.002
Miftahudin R, Miftahudin SH, Tatik C (2019) Antioxidant activity of ethanolic extract of three Selaginella species from Java Island, Indonesia. Biodiversitas 20(12):3715–3722. https://doi.org/10.13057/biodiv/d201234
Miller GL (1972) Use of dinitro-salicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Moore S, Stein WH (1949) Amino acid composition of ß-lactoglobulin and bovine serum albumin. J Biol Chem 178:79–91
Navari-Izzo F, Rascio N (1999) Plant response to water deficit conditions. In: Pessarakli M (ed) Handbook of plant and crop stress. Marcel-Dekker, New York, pp 231–270
Navari-Izzo F, Pinzino C, Quartacci MF, Sgherri CLM, Izzo R (1994) Intracellular membranes: kinetics of superoxide production and changes in thylakoids of resurrection plants upon dehydration and rehydration. Proc R Soc Edinburgh 102B:187–191
Nyachiro JM, Briggs KG, Hoddinott J, Johnson FAM (2001) Chlorophyll content, chlorophyll fluorescence and water deficit in spring wheat. Cereal Res Commun 29:135–142. https://doi.org/10.1007/BF03543653
Oliver MJ, Tuba Z, Mishler BD (2000) The evolution of vegetative desiccation tolerance in land plants. Plant Ecol 151:85–100
Oliver M, Guo L, Alexander D, Ryals J, Wone B, Cushman J (2011) A sister group metabolomic contrast using untargeted global metabalomic analysis delineates the biochemical regulation underlying desiccation tolerance in Sporobolus stapfianus. Plant Cell 23:1231–1248. https://doi.org/10.1105/tpc.110.082800
Patrick J, Mahony O, Melvin JO (1999) The involvement of ubiquitin in vegetative desiccation tolerance. Plant Mol Biol 41:657–667. https://doi.org/10.1023/a:1006330623364
Raza A, Razzaq A, Mehmood SS, Zou X, Zhang X, Lv Y et al (2019) Impact of climate change on crops adaptation and strategies to tackle its outcome: a review. Plants 8:34. https://doi.org/10.3390/plants8020034
Sanchez D, Siahpoosh M, Udvardi M, Kopka J (2008) Plant metabolomics reveals conserved and divergent metabolic responses to salinity. Physiol Plant 132:209–219. https://doi.org/10.1111/j.1399-3054.2007.00993.x
Schwab KB, Schreiber U, Heber U (1989) Response of photosynthesis and respiration of resurrection plants to desiccation and rehydration. Planta 177:217–227
Sharma L, Priya M, Kaushal N, Bhandhari K, Chaudhary S, Dhankher OP et al (2020) Plant growth-regulating molecules as thermoprotectants: functional relevance and prospects for improving heat tolerance in food crops. J Exp Bot 71:569–594. https://doi.org/10.1093/jxb/erz333
Sherwin HW, Farrant JM (1996) Differences in rehydration of three desiccation-tolerant angiosperm species. Ann Bot 78:703–710. https://doi.org/10.1006/anbo.1996.0180
Sherwin HW, Farrant JM (1998) Protection mechanisms against excess light in the resurrection plants Craterostigma wilmsii and Xerophyta viscosa. Plant Growth Reg 24:203–210. https://doi.org/10.1023/A:1005801610891
Sivaraman A, Johnson M, Parimelazhagan T, Irudayaraj V (2013) Evaluation of antioxidant potential of ethanolic extracts of selected species of Selaginella. IJNPR 4(3):238–244
Stewart CR (1981) Proline accumulation: biochemical aspects. In: Paleg LG, Aspinall D (eds) Physiology and biochemistry of drought resistance in plants. Academic Press, Sydney, pp 243–251
Suzuki N (2016) Hormone signaling pathways under stress combinations. Plant Signal Behav 11:e1247139. https://doi.org/10.1080/15592324.2016.1247139
Syaefudin JA, Rosiyana L, Setyani A, Khodijah S (2016) Nanoparticles of Selaginella doederleinii leaf extract inhibit human lung cancer cells A549 IOP Conf Series. Earth Environ Sci 31:012029. https://doi.org/10.1088/1755-1315/31/1/012029
Taji T, Ohsumi C, Iuchi S, Seki M, Kasuga M, Kobayashi M, Yamaguchi SK, Shinozaki K (2002) Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J 294:417–426. https://doi.org/10.1046/j.0960-7412.2001.01227.x
Tamara R, Gordana G, Maja L, Branka SC (2014) Effects of different light intensities, CO2 concentrations, temperatures and drought stress on photosynthetic activity in two paleoendemic resurrection plant species Ramonda serbica and R. nathalia. Environ Exp Bot 109:63–72. https://doi.org/10.1016/j.envexpbot.2014.08.003
Tommaso M, Anne W, Adriana B, Concetta V, Akira S, Celine MD (2007) Amino acid pattern and glutamate metabolism during dehydration stress in the ‘resurrection’ plant Sporobolus stapfianus: a comparison between desiccation-sensitive and desiccation-tolerant leaves. J Exp Bot 58(11):3037–3046
Tripathi AK, Gautam M (2007) Biochemical parameters of plants as indicators of air pollution. J Environ Biol 28:127–132
Tuba Z, Lichtenthaler HK, Csintalan Z, Nagy Z, Szente K (1996) Loss of chlorophylls, cessation of photosynthetic CO2 assimilation and respiration in the poikilochlorophyllous plant Xerophyta scabrida during desiccation. Physiol Plant 96:383–388. https://doi.org/10.1111/j.1399-3054.1996.tb00448.x
Tuba Z, Smirnoff N, Csintalan Z, Szente K, Nagy Z (1997) Respiration during slow desiccation of the poikilochlorophyllous desiccation tolerant plant Xerophyta scabrida at present-day CO2 concentration. J Plant Physiol Biochem 35:381–386
Tuba Z, Proctor MCF, Csintalan Z (1998) Ecophysiological responses of homoichlorophyllous and poikilochlorophyllous desiccation tolerant plant Xerophyta scabrica at present day CO2 concentration. Plant Physiol Biochem 35:381–386
Tymms MJ, Gaff DF (1979) Proline accumulation during water stress in resurrection plants. J Exp Biol 30:165–168
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759. https://doi.org/10.1007/s00726-008-0061-6
Wang X, Chen S, Heng Z, Lei S, Fenglin C, Lihai G, Yongming X, Tai W, Xiufeng Y, Shaojun D (2010) Desiccation tolerance mechanism in resurrection fern-ally Selaginella tamariscina revealed by physiological and proteomic analysis. J Proteom Res 9:6561–6577
Whittaker A, Bochicchio A, Vazzana C, Lindsey G, Farrant J (2001) Changes in leaf hexokinase activity and metabolite levels in response to drying in the desiccation-tolerant species Sporobolus stapfianus and Xerophyta viscosa. J Exp Bot 52:961–969. https://doi.org/10.1093/jexbot/52.358.961
Wiechert W, Schweissgut O, Takanaga H, Frommer W (2007) Fluxomics: mass spectrometry versus quantitative imaging. Curr Opin Plant Biol 10:323–330. https://doi.org/10.1016/j.pbi.2007.04.015
Wolters H, Jurgens G (2009) Survival of the flexible: hormonal growth control and adaptation in plant development. Nat Rev Genet 10:305–317. https://doi.org/10.1038/nrg2558
Yao H, Chen B, Zhang Y, Ou H, Li Y, Li S, Shi P, Lin X (2017) Analysis of the total biflavonoids extract from Selaginella doederleinii by HPLC-QTOF-MS and its in vitro and in vivo anticancer effects. Molecules 22(2):325. https://doi.org/10.3390/molecules22020325
Zhang Q, Song X, Bartels D (2016) Enzymes and metabolites in carbohydrate metabolism of desiccation tolerant plants. Proteomes 4:40. https://doi.org/10.3390/proteomes4040040
Zita K, Livia SS, Ildiko V, Gabor K (2012) Different accumulation of free amino acids during short- and long-term osmotic stress in wheat. Sci World J 10: Article ID 216521, 10 pages. https://doi.org/10.1100/2012/216521
Author information
Authors and Affiliations
Contributions
VK and MJ are equally contributed for the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kalaiarasi, V., Almeida, R.S., Coutinho, H.D.M. et al. Biochemical profile of resurrection fern allies: Selaginella wightii Hieron and Selaginella involvens (S.W.) Spring. Vegetos 35, 1014–1023 (2022). https://doi.org/10.1007/s42535-022-00371-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-022-00371-w