Abstract
An organic non-linear optical material of 4-methylanilinium 3,5-dinitrobenzoate (MADNBA) was synthesized and crystals of MADNBA were grown by employing slow evaporation solution growth technique. The crystal structure was confirmed from Single crystal XRD. Grown crystals were characterized by Powder XRD, UV–Vis–NIR, Photoluminescence and Laser damage threshold measurement studies. Infrared spectroscopy, thermal analysis and microhardness measurement were performed to study the molecular vibration, thermal behaviour and mechanical stability of the grown crystal. Dielectric studies have been performed to evaluate various solid state parameters. From the Kurtz Perry powder technique, it was found that Second harmonic generation efficiency of the obtained compound is 10 times that of the standard potassium dihydrogen phosphate. Z-Scan technique was also carried out to investigate the non-linear activity and optical limiting behaviour of MADNBA crystal. Computational analysis performed upon the optimized geometry of MADNBA molecule using Density Functional Theory confirmed the experimentally determined quantities under a strong theoretical background. Frontier molecular orbital analysis and molecular electrostatic potential analysis were carried out. The non-linear character of the compound was also determined by computing various non-linear optical parameters like dipole moment, polarizability and hyperpolarizability. The first order hyperpolarizability (β) value of the MADNBA molecule was evaluated to be 8 times that of the standard urea molecule. Based on these results, it could be proposed that MADNBA material serves as a potential candidate for frequency doubling, optical limiting and photonic applications in optoelectronics domain.
Similar content being viewed by others
1 Introduction
Recent research reveals that, organic materials with aromatic rings having less optical absorption, large band gap, high thermal as well as mechanical stability, less fast response with tailor-made flexibility and higher order of nonlinearity find wide applications in high resolution spectroscopy, laser printing, remote sensing, chemical and biological species detection and optical communication systems [1, 2]. Among those, material exhibiting high optical nonlinearity is considered to be a promising aspirant due to its potential usage in nonlinear optical technologies demanding low power laser systems [3, 4]. The synthesis and growth of such nonlinear optical materials seems to be a challenge in the fast developing fields of optoelectronics and photonics [5,6,7]. Within the organic category, dinitrobenzoate derivatives play a vital role in biological area due to its anti-creatinine property and these derivatives serves as radiation sensitizers for the treatment of tumour [8]. Apart from the biological applications, the role of dinitrobenzoate derivatives in the optoelectronics and photonics domain remains unexplored till now. Hence, in the present investigation we have been testing for the first time the non-linear character of a dinitrobenzoate derivative, 4-methylanilinium 3,5-dinitrobenzoate (MADNBA), utilizing various linear and non-linear optical characteristics and thereby confirms the non-linear activity of the compound demanded by various non-linear optical applications in optoelectronics technologies.
Present work deals with the interaction of 3,5-dinitrobenzoic acid ester with 4-methylanilinium and the formation of intermolecular N–H….O hydrogen bonding between the donor ammonium groups and the O atoms of the acceptor 3,5-dinitrobenzoate anions gives rise to a promising organic compound namely 4-methylanilinium 3,5-dinitrobenzoate (C7H10N+–C7H3N2O6−) [9, 10]. Strong hydrogen bond which serves as an imperative tool of stability occurs due to the interaction of negatively charged oxygen atom with the polarizable hydrogen atom covalently bonded to the positively charged nitrogen atom and this paves the way to increase the molecular hyperpolarizability [11]. Theoretical calculations are also adopted on the present molecule of 4-methylanilinium 3,5-dinitrobenzoate (MADNBA) in order to verify the experimentally determined quantities, reflecting the linear and non-linear optical characteristics of the compound. The most stable geometry of the title compound is illustrated in Fig. 1.
2 Experimental procedure
2.1 Material synthesis
All the reagents were purchased from Sigma Aldrich and used without further purification. The title compound,4-methylanilinium 3,5-dinitrobenzoate (MADNBA) was obtained by the addition of ethanolic solution of 4.66 g(0.022 mol)of 3,5-dinitrobenzoic acid to a solution of 2.14 g(0.02 mol)of 4-methylaniline in ethanol keeping 1:1 as the stoichiometric ratio. The mixture was stirred continuously for 4 h to obtain a homogeneous solution at room temperature. The light yellowish transparent solution thus obtained was filtered off using Grade:1 Whatmann filter paper and the filtered solution was kept at room temperature in a dust free atmosphere for slow evaporation. After 2 days, good quality pale yellowish coloured seed crystals were obtained and the dimension was further increased by successive re-crystallization from ethanol.
2.2 Solubility of the material
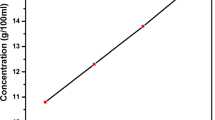
In the crystallization process, certain factors like temperature, solubility and super saturation serves as driving forces in optimizing the growth rate, dimension and quality of the crystal [12, 13]. The solubility study was performed in the temperature range of about 30–65 °C with ethanol and water as the solvent. Initially, MADNBA salt was added to 100 ml of ethanol taken in a container which is placed in a temperature controlled water bath of ± 0.01 °C accuracy attached with a magnetic stirrer. The solution was stirred at 30 °C for about 5 h to attain the homogeneity throughout the volume of the solution to ensure super saturation. Then by employing gravimetric method, 4.57 g of the synthesized salt was found to be dissolved in 100 ml of ethanol and 2.92 g in 100 ml of water at the super saturated state of 30 °C. The same procedure was done for 35, 40, 45, 50, 55, 60 and 65 °C and the corresponding amount of the salt getting dissolved with respective temperature was noted. The solubility curve of MADNBA compound is demonstrated in Fig. 2a which holds positive temperature co-efficient.
2.3 Crystal growth
On the basis of the solubility study, the saturated solution of MADNBA compound was prepared at 30 °C choosing ethanol as the appropriate solvent. The resultant super saturated homogenous solution was filtered successively to remove the suspended impurities and transferred to a crystal growth vessel covered with polythene sheet of limited holes. The crystal growth vessel was then kept in a dust free atmosphere at room temperature to facilitate the nucleation process. Some good quality seed crystals were harvested within a short span of 5 days and those crystals were hung in the parent solution for obtaining the bulk crystals of required dimension. Large crystals of dimension 10*4*3 mm3 (Fig. 2b) were harvested within 2 weeks and this superior optical quality bulk crystal was subjected for different linear and non-linear optical characterizations.
2.4 Characterization techniques
Single crystal X-ray diffraction (XRD) analysis was carried out employing ENRAF NONIUS CAD4 diffractometer for computing the structural parameters and Powder X-ray diffraction (PXRD) spectrum was obtained by Cary 630 with ATR- Agilent technologies to ensure the crystalline nature of the grown MADNBA single crystal. Fourier Transform Infrared spectroscopy (FTIR) was performed using Rigaku Miniflex 600 set-up for identifying the various functional groups embedded within the grown crystal. Thermo gravimetric and Differential Scanning Calorimetric (TG&DSC) profiles were formed by utilizing NETZSCH STA 449F5 STA449FSA-0231-M thermal analysis system to confirm the thermal stability of MADNBA material. UV–Vis-NIR and fluorescence spectra were formed by Varian Carry 5000 spectrophotometer and Shimadzu Spectrofluorophotometer to determine the transluscence nature and luminescence property of the grown crystal. LEITZ WETZLER micro hardness tester with pyramidal indenter was handled to evaluate the mechanical stability of the material, dielectric studies were done through HIOKI 3532 LCR HITESTER set-up. The second and third order non-linear behaviour of the material was verified through Kurtz Perry powder technique and Z-Scan technique.
3 Results and discussion
3.1 Crystallographic studies
The grown single crystal of MADNBA was subjected to X-ray diffraction studies and the crystal diffractogram was further analysed using ENRAF NONIUS CAD4 software to determine the lattice dimensions of the obtained compound. The lattice parameters were estimated by least-squares fitting to the setting angles of 25 reflections occurred in diffractogram [14]. It was found that the crystal belongs to orthorhombic system with non-centrosymmetric space group Pbca and all other cell parameters was found in good agreement with the reported values,Footnote 1 as tabulated below (Table 1);
The obtained crystal was powdered and exposed to powder XRD to confirm the phase purity and the crystalline nature of the material and the X-ray diffraction pattern is depicted in Fig. 3.
Various structural parameters like crystallite size, dislocation density, micro strain within the crystal lattice and degree of crystallinity of the grown MADNBA single crystal can be evaluated from the Powder XRD pattern following some mathematical formulations [15].
The crystallite size of MADNBA can be computed using Debye–Scherrer’s formula;
where the constant, k = 0.9, λ = 1.5405 Å & β exhibits the full width at half maximum value. The X-ray diffraction spectrum helps us to identify that, the maximum intensified peak was found to be at 2θ = 19.79°. The size of MADNBA crystal was then calculated to be 6.75 Å. Later on, the crystallite size was observed to be in good agreement with the result of Hall Williamson plot shown in Fig. 4. The dislocation density of the MADNBA crystal lattice was obtained from the expression;
The occurance of microstrain was then verified using the Hall Williamson co-relation;
The evaluated value of microstrain within MADNBA crystal lattice exactly matches with the slope of the Hall–Williamson plot. Additionally, the percentage of crystallinity of the grown crystal was also calculated to verify the crystalline nature of the obtained compound. The obtained crystallographic parameters of the grown crystal of MADNBA are depicted in Table 2.
3.2 Ftir analysis
FTIR spectroscopy is an effective tool to identify and to confirm a compound by analysing the functional groups present within the spectrum. The molecular interactions between the reagents can be well explained by examining different modes of vibrations and deformations like stretching, wagging, bending, rocking and scissoring. Fourier Transform Infrared analysis of the material is carried out by employing FTIR spectrometer using KBr pellet technique. The FTIR spectrum of the synthesized material, MADNBA is recorded in the region of 500–4000 cm−1 and is depicted in Fig. 5. The existence of weak intensified peak at 3091.31 cm−1 represents the stretching vibration of C–H group and the symmetric stretching mode of CH3 molecule is exhibited by the peak at 2624.42 cm−1. The peak observed at 1887.58 cm−1 is related to the in-plane deformation of NH2 scissoring mode of C–C stretching vibration. The O–H bending character due to the intermolecular hydrogen bonding and in-plane deformation of CH3 symmetric mode is noticed through the energy transfer corresponding to 1627.64 cm−1 and 1532.66 cm−1. The symmetric stretching vibration of nitro groups and C–O groups are strictly exhibited by peak depicted in 1342.70 cm−1. Peaks identified at 1151.74 cm−1 and 1073.76 cm−1 shows the presence of out of plane deformation of CH3 group and in-plane deformation of methyl groups. The out of plane deformations of C–H bending modes in symmetric tri substituted benzene derivatives and NH2 wagging modes originates from 909.80 to 796.82 cm−1. The sharp intensified peak noticed at 718.84 cm−1 reflects the radial skeletal vibrations of symmetric tri substituted 3,5-dinitrobenzoic acid acids and the out of plane deformations of C–CH3 modes. The in-plane deformations of NO2 group and out of plane deformations coupled with wagging vibrations of amino group is identified by sharp peaks at 527.88 cm−1, 484.89 cm−1 and 406.91 cm−1.
3.3 Thermal exploration
The Thermogravimetric(TG) and Differential Scanning Calorimetric(DSC) profiles of MADNBA (Fig. 6) was analysed to verify the possibility of using the crystalline material for non-linear optical applications. Because the thermal stability and physiochemical properties of the sample has to be strictly encountered since the NLO devices fabricated with the obtained compound will generate enormous amount of heat when subjected to polishing, cutting and while being exposed to continuous wave laser [16]. The MADNBA compound weighing 12.02 mg is heated between 30 °C and 500 °C at a heating rate of 10 K/min under nitrogen atmosphere. The TG trace exhibits two stage weight loss patterns with no significant loss up to 128 °C, which is attributed to the melting point of the sample. The first stage of weight loss was observed within the temperature range 129–342 °C with a major mass loss of about 57.43% followed by the second weight loss of about 14.24% occurring between 343 °C and 500 °C. The remaining percentage of mass exists as the residual mass of carbon atoms. From the DSC curve, it can be inferred that the decomposition of the material starts at 149 °C and it can be confirmed by the sharp endothermic peak in the DSC trace which coincides with that of the melting temperature range in the TG curve. The decomposition of the compound is correlated with the liberation of the volatile gases like CO2, NO2, NH2 etc. The sharpness of the peak is mainly attributed to the purity and the crystallinity of the sample. Decomposition of the various functional groups present in the obtained compound is illustrated by the DSC data which is in good agreement with the second stage of weight loss shown in the TG profile. Based on the thermal studies, MADNBA can be considered as a potential candidate for NLO applications operating under a maximum utility temperature of about 128 °C.
3.4 UV–VIS–NIR spectral analysis
The grown crystal of 4-methylanilinium 3, 5-dinitrobenzoate dissolved in ethanol was subjected to spectral studies within 200–800 nm wavelength range. The optical characteristics usually exhibits the absorption bands corresponding to π–π*or n–π* transitions within the materials [17]. In short, absorption as well as transmission spectra helps us to identify the nature of the molecule by analysing the transition of electrons in σ and π orbitals between ground state and higher excited states with the energy absorbed [18]. The obtained optical transmission spectrum is as shown in Fig. 7. The fundamental absorption band was observed at 316.56 nm, which is exploited for the excitation of electrons from the valence band to the conduction band [17]. This weak absorption edge may refer to the n–π* promotions and the transition occurring within the aromatic ring in the sample [17]. The existence of enhanced optical transparency in the Vis–IR region enables the material to be used as a promising aspirant for higher order of non-linear optical generations and terahertz applications [19]. The lower cut-off wavelength (316.56 nm) followed by wide optical transparency in the UV–Vis–NIR region promotes the material for the generation of second harmonic waves(532 nm) and third harmonic waves (355 nm) from the input source of 1064 nm wavelength [20].
3.4.1 Determination of optical band gap energy
The property of electronic optical band gap energy can be well exploited in the fields of terahertz generation and electro-optical device applications, since it provides some basic understanding of the electronic and atomic band structures [21]. Assuming parabolic nature, the optical absorption co-efficient near the absorption edge (α) and Eg holds the relation [22];
where ‘h’ is the Planck’s constant, ‘ν’-frequency of vibration, ‘Eg’- optical band gap, ‘A|’- a proportionality constant and the exponent ‘n’ reflects the nature of the sample transition [23]. In the present work, ‘n’ is assumed to be 2, because only the direct allowed transition is accounted in MADNBA sample.
According to Tauc et al., the direct optical band gap energy was calculated by evaluating the optical co-efficient(α) from the percentage of transmittance recorded by the spectrum, via the expression;
here ‘A’ is the absorbance and‘t’ is the thickness of the crystal.
Hence the optical band gap energy can be determined from the Tauc’s plot between (αhυ)2 and the photon energy hυ (eV) as exhibited in Fig. 8. Then, by extrapolating the linear portion of Tauc’s plot to the axis where (αhν)2 = 0, the value for optical band gap can be obtained. The Band gap energy was evaluated to be 3.92 eV, which is in good agreement with the band gap energy evaluated theoretically by the equation;
where ‘λ’ denotes the absorption wavelength. This higher value of band gap energy reflecting the wide optical transparency in the UV–Vis–NIR region, strictly confirms the promising candidature of MADNBA crystal to be used for UV tunable lasers and opto-electronic laser applications [24].
The optical properties like refractive index of MADNBA (n), optical reflectance (R), electric susceptibility (χc) of the material and the Urbach energy (Eu) can be found using the following formulations;
Urbach rule can be defined as the process of tailing of the density of states of MADNBA material, into the forbidden band gap during the transition of electrons between the valence band and the conduction band. The phenomenon of Urbach rule can be related to photon energy as;
where α0 is the proportionality constant, hν, the photon energy and Eu is the respective Urbach energy. Urbach energy can be explained as the infinitesimal amount of energy which serves as a key factor in determining the structural disorder and defects within the MADNBA material [25].
Taking logarithm on both sides;
The Urbach energy can be obtained as the reciprocal of the graph plotted between lnα and hυ (Table 3).
3.5 Fluorescence studies
Photoluminescence is the spontaneous emission of radiation when a material undergoes optical excitation. Fluorescence study is a prominent tool to determine the physical properties of a material at molecular level, including crystallinity and purity. Organic compounds containing aromatic structures with lowest energy π-π* transitions are expected to exhibit high luminescence intensity [26, 27]. Fluorescence spectrum was obtained by Shimadzu Spectrofluorophotometer with the sample excited at an excitation wavelength of 316 nm and the spectrum was recorded in the range of 450–850 nm (Fig. 9). The material showed a sharpened peak at 620 nm indicating Red emission with the maximum intensity. A higher intensity ratio in the luminescence spectrum is an evidence of better purity, crystallinity and structural perfection of the MADNBA compound, hence this organic molecule with promising fluorescence emission can be exploited for NLO applications [10, 28].
3.6 Microhardness analysis
Mechanical parameters of a crystal like hardening co-efficient, stiffness constant and yield strength plays a key role in device fabrications and robust photonic applications to estimate the chemical composition, deformation characteristics as well as plasticity/elasticity of the material [29,30,31,32]. The hardness behaviour of a material is highly influenced by the debye temperature and space lattice energy existing between the atoms [33, 34]. The mechanical strength of the MADNBA sample is manipulated by employing Vicker’s microhardness test. For the indentation test, a well-polished surface of the crystal sample was subjected to different loads in the order of 20–100 gm at constant indentation time of 10 ns. The variation in hardness nature corresponding to applied loads is depicted in Fig. 10. It is evident that the hardness number increases with the increase in external load obeying Reverse Indentation Size Effect (RISE) [35]. But beyond a load of 50 gm, it is found to be decreasing with the load due to frictional and elastic behaviour of the material [35] divulging normal Indendation Size effect [36,37,38].
The Vicker’s hardness number (Hv) can then be evaluated using the relation;
where ‘P’ and ‘d’ denotes the applied load in gm and the average diagonal length of the indenter during the impression on the crystal surface.
The reverse indentation size effect is shown by the crystal up to 50 gm because the diamond pyramidal indenter has pierced only to the softer layers of MADNBA initially, but beyond that load an internal stress has released forming cracks within the crystal lattice attributing to normal indentation size effect.
The nature of the material, stiffness constant reflecting the bonding strength in atoms and the yield strength exhibiting the elastic nature of materials can then be computed probing the applied load with the length of indentation and Hardness value.
Onitsch and Hanneman proposed that Meyer’s law can be interpreted as;
Here ‘a’ is the constant of proportionality and ‘n’ is termed as the Meyer’s index. The above equation can be logarithmically interpreted as;
By plotting a graph between logd and logP (Fig. 11), the slope of the straight line curve provides us the Meyer’s index or work hardening co-efficient. This exponent factor decides the nature of the material. As per the theory proposed by Onstich and Hanneman, the crystal will be honoured as hard material when n < 1.6 and as soft material when n becomes greater than 2 [39, 40].
The bonding nature in atoms can then be defined by the Wooster’s empirical formula;
Higher the value of C11, strong will be the binding strength between ions.
The plastic deformation in the crystal lattice due to the internal stress can be evaluated exploiting the relation;
The corresponding variations in stiffness constant (C11) and yield strength (σy) with respect to the applied load (P) is shown in Fig. 12a, b (Table 4).
3.7 Dielectric studies
Non-linear optical applications are strongly influenced by dielectric analysis since it provides basic information about the electrical polarizable effect in materials by the externally applied electric field [41]. Dielectric constant can be defined as the capability of the material to accumulate charge in the presence of electromagnetic field. On the basis of the principle, opposite faces of the MADNBA crystal of thickness 0.89 mm was polished and silver layered for forming a parallel plate capacitor. Then the study was performed to evaluate the parallel capacitance (Cp) within 1 Hz–1 MHz frequency range, employing temperature states of 33 °C, 50 °C and 70 °C. The value of dielectric constant is then accessed from the equation;
The frequency dependent variation of dielectric constant is plotted in Fig. 13. From the figure, it can be inferred that at low frequency, dielectric attains a maximum value and it decays exponentially up to 10 kHz with the increase in frequency and reaches a constant value at higher frequency to about 51.27. The higher value of εr at low frequency and higher temperature reflects the presence of space charge, ionic, orientation and electronic charge polarization due to the impedance offered by the parallel plate capacitor to the motion of charge carriers between the electrode and it is attributed to the perfection of the MADNBA crystal [42,43,44]. The value of dielectric constant saturates and attains a constant value at higher frequency and it reveals the diminishing nature of the exhibited polarizations with the introduction of dipole rotation. This property of the dielectric behaviour promotes to the better optical quality of MADNBA crystal with fewer defects and dislocations which can be utilized in high speed integrated laser applications and plays a vital role in SHG process [45].
3.7.1 Determination of solid state parameters
The electro-optic polarizability serves as a key parameter in explaining the contribution of optical non-linearity in materials. Here the theoretical approaches of Penn gap analysis and Clausius–Mosotti relation is incorporated to obtain electronic polarizability (α) for MADNBA crystal in the high frequency region [46]. The various non-linear parameters are derived purely based on the empirical formula of MADNBA crystal, whose M = 319.27 g/mol, ρ = 2.17 g/cm3 and total number of valence electrons (z) = 126.
Using the above mentioned characteristics, plasma energy can be measured via the formula;
where ωp is the plasma angular frequency.
From the above formalism, Penn gap Ep and Fermi energy Ef can be related as;
Hence the polarizability(α) of MADNBA crystal can be computed from Penn gap energy employing the relation;
where So is a constant for any material expressed as,
The non-linear property of electronic polarizability, α can also be determined from the Clausius–Mossotti equation, which is co-related by;
where Na is the Avogadro number. The obtained solid state parameters are tabulated in Table 5 and compared with KDP values for establishing the non-linear nature of the material. The enhanced polarizability value symbolizes the promising non-linear behaviour of MADNBA single crystal.
3.8 Laser damage threshold study
For a material to be used in non-linear optical applications like frequency doubling phenomenon, we should take into account the surface quality/threshold capacity of the crystal towards high power laser intensities [47]. Laser damage threshold measurement was carried out to verify the efficiency of the optical component in materials to resist the incoming radiation. It mainly depends on the dislocation density of the crystal i.e., lesser the dislocation; higher will be the threshold value which denotes higher laser stability. The study was performed on MADNBA crystal of 0.65 mm thickness using Q-Switched pulsed Nd-YAG laser of 1064 nm delivering 9 ns pulses at repetition rate of 10 Hz. A lens of 10 cm focal length was employed for converging the laser beam and the input energy corresponds to the surface damage was recorded using the power meter. Initially, MADNBA crystal was subjected to 20 mJ of energy intensity and then the range of energy were further increased from 21 to 32 mJ. Finally, a surface damage was observed with the incident intensity of 33 mJ, which is noted as the maximum incoming laser energy that can be tolerated by the crystal surface of MADNBA. The corresponding power density was computed through the co-relation;
Here ‘E’ is the input energy in mJ, the energy with which the crystal undergoes damage, ‘τ’, the pulse width in ns and the parameter ‘A’ serves as the area of the circular spot (πr2), observed on the crystal surface.
The evaluated multiple shot LDT value was estimated to be 10.81 GW/cm2. It is 54 & 7.2 times higher than that of the typical NLO crystals, KDP and Urea. The promising LDT value of MADNBA pronounces its resourceful candidature in high power laser fabrications and opto-electronic applications (Table 6).
3.9 Second harmonic generation studies
The non-linear optical activity of the grown crystal is investigated through Kurtz–Perry powder technique by performing the SHG test [48]. The frequency conversion efficiency of a material strongly blends with the impurities, cationic defects and the intermolecular charge transfer interaction within the crystal lattice [49]. In the present analysis, the grounded microcrystalline sample of MADNBA crystal taken in a capillary tube was exposed to the continuous wave mode locked Q-switched Nd-YAG laser of fundamental wavelength 1064 nm delivering 6 ns laser pulses following a repetition rate of 10 Hz. When the sample is irradiated to a laser input of 5.5 mJ, an SHG signal of 0.75 V voltage was obtained as the output of green emission. The same procedure was repeated for KDP crystals powdered uniformly to the grinded size of MADNBA crystals and the output voltage was recorded by the photo detector as 75 mV. Hence the SHG efficiency of the grown crystal of MADNBA was observed to be 10 times that of the typical NLO crystal KDP. The enhanced NLO efficiency is strongly influenced by the significant proton transfer interaction took place between the aniline moiety and benzoic acid moiety of the methylaniline group and dinitrobenzoic acid group as well as due to the growth of defect free MADNBA crystal lattice. All the above mentioned results confirm the non-centrosymmetric structure of MADNBA satisfying the perquisites to be followed by every NLO material for accommodating in photonic applications (Table 7).
3.10 Z-scan measurement
In the present scenario, the third order non-linear activity in MADNBA crystal is studied by utilizing the open aperture Z-Scan technique [55]. For the study, Intensity variations of incoming laser beam with the translation of sample is considered as the imperative tool for evaluating the optical property of the material. In the open aperture Z-Scan approach, Q-Switched Nd-YAG laser producing frequency doubling output of 532 nm and pulse width of 5 ns was used to excite the sample. The sample is translated through the z-direction and the transmittance is measured as the function of sample position with respect to the beam focus (z = 0). The obtained open aperture z-scan trace with an input energy of 80 µJ is illustrated in Fig. 14. The plot symbolizes asymmetric valley about the focus(z = 0) which indicates the Reverse Saturable Absorption (RSA) effect due to Two Photon Absorption (TPA) process, which can be explained as the higher absorption of excited state when compared with the ground state absorption [56]. Even though TPA phenomenon dominates in the non-linear behaviour of material, Saturable Absorption (SA) effect should also be accumulated along with RSA effect for obtaining accuracy in the non-linear optical activity of MADNBA crystal. Hence the net non-linear absorption co-efficient (α) can be accessed using the parameters, β for RSA effect and Is representing SA effect as follows;
Here α0 is represented by the unsaturated linear absorption co-efficient and β is termed as the two photon absorption co-efficient or the excited state absorption co-efficient. These quantities can be determined by fitting the experimentally plotted open aperture z-scan curve with the pulse propagation equation given by;
where z| is the propagation distance. By the accurate fitting of the numerical equation on the experimentally obtained curve, the values of β and Is was determined to be 0.58 × 10−11 m/W and 15 × 1012 W/m2.

3.10.1 Optical limiting property
The optical power limiting is a non-linear effect in which the transmittance reaches threshold intensity at a particular input energy and thereby clamping and guarding the optical sensors and human eyes from getting damaged of highly intensified laser light. The property of optical limiting can be achieved through various non-linear parametric processes like multi photon absorption (MPA), Excited state absorption (ESA), self-focussing/de-focussing and optically induced phase transitions [57]. The optical limiting curve is depicted in Fig. 15 with the transmittance energy as a function of input fluence. The input laser energy corresponding to different z-position is accessed through the relation [58];
where Ein is the energy of the input laser light.
From the curve, it is understood that the transmitted intensity of laser beam attains a saturated value at 7.084 J/cm2 and starts decreasing gradually with the further increase in input energy. This saturated value is termed as the optical limiting amplitude of the MADNBA material. Lower threshold value of 7.084 J/cm2 pronounces the title compound to be used as an efficient optical limiter in extensive fields of optical sensor protection, pulse compression and passive mode-locking applications [59].
3.11 Computed molecular geometry
3.11.1 DFT calculations
The optimized geometry of MADNBA molecule was analysed by the finite field theory method of Density Functional Theory, DFT-B3LYP/6-31 ++G*d(p) with Gaussian 09 W software package. Frontier Molecular Orbital (FMO) analysis and Molecular Electrostatic Potential (MESP) analysis was performed on MADNBA molecule to verify the experimentally determined optical band gap energy and the occurance of charge transfer mechanism within the molecule. Additional insight was provided on the non-linear behaviour of the molecule by evaluating dipole moment, polarizability and hyperpolarizability parameters using DFT-B3LYP method and ab initio Hartee–Fock (HF) functionals with the same basis set of 6–31 ++G*d(p) for the ease of comparison. The ground state molecular structure of the MADNBA compound was obtained using Chemcraft software and is depicted in Fig. 16a [60].
3.11.2 Frontier molecular orbital (FMO)/HOMO–LUMO analysis
Frontier molecular orbital (FMO) analysis exists as the crucial factor in quantum chemistry predicting the reactive groups in the π electron conjugated molecular system. The FMO’s utilizes the concept of highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) (Fig. 16b) as the key parameters in predicting the significance of intermolecular charge transfer interaction through the π conjugated path of donor and acceptor groups within the MADNBA molecule. In the present study, aniline moiety over the methylaniline group acts as the electron donor whereas hydrogen atoms on the benzoic acid moiety in the dinitrobenzoic acid group play the role of electron withdrawer. Hence the HOMO–LUMO transition indicates the protonation from the 4-methylaniline cation to the 3,5-dinitrobenzoic acid anion with the formation of intermolecular hydrogen bonds. The estimated HOMO–LUMO energy gap reflects the presence of a strong energy barrier for the electron density transfer between the donor and acceptor groups which paves the way for higher stability and adequate chemical hardness for the MADNBA molecule. It was found that the one electron excitation between π–π* transition state is occurring through the HOMO–LUMO interaction [61]. The lowest value of HOMO and LUMO energies discloses the higher stabilization of LUMO with the destabilization of HOMO which enhances the NLO behaviour of the material influenced by the strong hydrogen bond interaction and extended π conjugations.
3.11.3 Molecular electrostatic surface potential (MESP) analysis
The three dimensional visualization of molecular electrostatic potential mapping (MESP) illustrates the atomic charge distribution on the MADNBA molecular structure by figuring out the electrophilic and nucleophilic sites extended over the surface of the molecule. The surface diagram as shown in Fig. 16c provides the information of reactive sites in terms of colour grading mechanism. The molecule with red colour represents the most negative potential encouraging electrophilic attack and the molecules holding blue colour favours nucleophilic attack since it has positive potential signature. Here in MADNBA molecule, the most attractive (−ve potential) electrostatic potential region is exhibited by methylaniline group as well as the most repulsive (+ve potential) electrostatic potential site is demonstrated by dinitrobenzoic acid group and those regions are considered as the preferable sites for electrophilic and nucleophilic attack. The regions represented in green colour reflect the zero potential area which primarily acts as the medium of interaction.
3.11.4 Dipole, polarizability and hyperpolarizability calculations
Organic molecules with delocalized π conjugated electronic system are counted as the promising NLO candidates, since they generate large molecular hyperpolarizability through the efficient charge transfer mechanism with the aid of π conjugated bridge framework and push–pull structure [62,63,64]. The density functional theory (DFT) was employed for calculating various non-linear optical parameters like dipole moment, polarizability and dynamic (hyper) polarizability for evaluating the strength of proton transfer interaction. The finite field method of computational analysis is exploited for determining the NLO quantities accessing the following relationship;
where \(\beta_{x} = \beta_{xxx} + \beta_{xyy} + \beta_{xzz}\),\(\beta_{y} = \beta_{yyy} + \beta_{yxx} + \beta_{yzz}\)
And \(\beta_{z} = \beta_{zzz} + \beta_{zxx} + \beta_{zyy}\)
Second order hyperpolarizability,
The electric dipole moment (μ) of MADNBA molecule was computed to be 3 times when compared with the standard urea molecule (μ = 2.3732D) and the first order hyperpolarizability (β) was found to be 8 times that of urea (β = 0.3728 × 10−30 esu) which confirms the proton transfer interaction within the precursor molecules. The optical band gap energy of the MADNBA molecule was estimated as 3.112 eV. A slight deviation was observed in the band gap energy values because theoretical calculation is carried out upon 2D geometry of the molecule in gaseous environment as well as all experimental characterizations was subjected with the 3D crystal structure of the compound under solid state background. The large value of dipole moment and hyperpolarizability strictly establishes the structure property relationship holding the hydrogen bonding mechanism between the aniline cation and benzoic acid anion which could also contribute to the NLO property of the MADNBA molecule (Tables 8, 9).
4 Conclusion
Optically good quality single crystals of 4-methylanilinium 3,5-dinitrobenzoate (MADNBA), an organic non-linear optical crystal was synthesized and grown by the conventional slow evaporation solution growth technique. The crystallographic studies were carried out using the Single XRD structural pattern and found that MADNBA crystal belongs to orthorhombic system with Pbca as the non-centrosymmetric space group. Most prominent properties of a non-linear material like crystallinity and purity are strictly confirmed via Powder XRD spectrum. The functional groups of the MADNBA crystal have been detected by FTIR spectroscopy. The thermal and the mechanical stability of MADNBA compound was verified using TG/DSC analysis and Vickers micro hardness test and confirmed its potential utility up to 128 °C. UV–Vis–NIR spectrum confirms the wide optical transparency of the compound from UV to NIR region of wavelength with optical band gap energy of 3.92 eV, which ensures the ability of the material to generate higher harmonic frequencies in non-linear optical applications. The luminescence property of the material was examined from Fluorescence studies with an emission peak at 620 nm exhibiting its utility in red light emitting diodes. The non-linear property of electronic polarizability were evaluated exploiting dielectric studies. The frequency conversion efficiency of MADNBA crystal was estimated to be 10 fold of standard KDP and the surface damage threshold of 10.81 GW/cm2 reveals the practical usability of MADNBA material for second harmonic generations and high power laser-assisted optoelectronic applications. Third order non-linearity of the title compound was determined using Z-Scan technique and the lower optical limiting threshold of about 7.084 J/cm2 reflects the promising candidature of MADNBA material as efficient optical limiters. Quantum chemistry approach was adopted for computing the HOMO–LUMO energy gap, molecular electrostatic potential mapping and various non-linear optical parameters like dipole moment (µ), polarizability (α) and hyperpolarizability (β). The electric dipole moment (µ) and first order hyperpolarizability (β) was obtained to be 3 times and 8 times that of the standard reference material of urea which strictly confirms the non-linear activity of MADNBA molecule. Based on the above characterizations, this novel NLO crystal of MADNBA could be proposed for opto-electronic device fabrications and non-linear optical applications.
Notes
Details regarding synthesis and the reported Single XRD of the grown MADNBA single crystal: Rui-jun Xu, Acta Crystallographica Section E. E66(2010)o1574. (http://doi.org/10.1107/s1600536810018441).
References
Subhasini A, Kumaravel R, Leela S, Evans HS, Sastikumar D, Ramamurthi K (2011) Synthesis, growth and characterization of 4-bromo-4|-chloro benzylidene aniline- A third order non linear optical material. Spectrochim Acta Part A Mol Biomol Spectrosc 78:935–941
Suresh M, Asath Bahadur S, Athimoolam S (2013) Crystal growth and characterization of a new NLO material: 4-Methoxyaniline. Pelagia Res Libr 4(4):160–164
Sathiya S, Senthikumar M, Ramachandra Raja C (2019) Crystal growth, Hirshfeld surface analysis, DFT study and third order NLO studies of thiourea 4 dimethyl aminobenzaldehyde. J Mol Struct 1180:81–88
Naseema K, Rao V, Sujith KV, Kalluraya B (2010) Crystal growth and characterization of an NLO organic crystal: N′-[(Z)-(4-methylphenyl)methylidene]-4-nitrobenzohydrazide. Curr Appl Phys 10:1236
Ravi S, Sreedharan R, Raghi KR, Kumar TM, Naseema K (2020) Experimental and computational perspectives on linear and non-linear optical parameters of an orthorhombic crystal for optical limiting applications. Appl Phys A Mater Sci Process 126:56
Naseema K, Rao V, Manjunatha KB, Umesh G, Sujith KV, Kalluraya B (2010) Synthesis, characterization and studies on nonlinear optical parameters of 4-amino-5-(4-nitrophenyl)-1, 2, 4-triazole-3-thione. J Opt 39:143
Naseema K, Ravi S, Sreedhran R (2019) Studies on a novel organic NLO single crystal: L-asparaginium oxalate. Chin J Phys 60:612–622
Beaula TJ, Muthuraja P, Dhandapani M, Jothy VB (2018) Effect of charge transfer with spectral analysis on the antibacterial compound 4(Dimethyl amino) pyridine: 3,5-Dinitrobenzoic acid: Experimental and theoretical perspective. J Mol Struct. https://doi.org/10.1016/j.molstruc.2018.06.026
Xu R-J (2010) 4-Methylanilinium 3,5-dinitrobenzoate. Acta Crystallogr Sect E E66:1574. https://doi.org/10.1107/S1600536810018441
Alam K, Khan IM (2018) Crystallographic, dynamic and Hirshfeld surface studies of charge transfer complex of imidazole as a donor with 3,5-dinitrobenzoic acid as an acceptor: determination of various physical parameters. Org Electron. https://doi.org/10.1016/j.orgel.2018.08.037
Sathya K, Dhamodharan P, Dhandapani M (2018) Structural characterization and DFT study of a new optical crystal: 2-amino-3-methylpyridinium-3,5-dinitrobenzoate. Opt Laser Technol 101:328–340
Bhuvaneshwari R, Vinitha G, Murugesan KS (2019) Crystal growth, optical, physico-chemical and third-order nonlinear optical studies of morpholinium oxalate: a new organic single crystal for optical limiting application. Appl Phys A Mater Sci Process. https://doi.org/10.1007/s00339.019.2678-6
Ramesh KS, Saravanabhavan M, Rajikumar M, Edison D, Sekar M, Muhammad S, Al-sehemi AG (2019) Synthesis, growth, structural, thermal, third order nonlinear and computational studies of organic single crystal: 2-Amino-4-picolinium 2chloro-4-nitrobenzoate. Opt Mater 96:109341
Magesh M, Bhagavannarayana G, Ramasamy P (2015) Synthesis, crystal growth and characterization of an organic material: 2-Aminopyridinium succinate succinic acid single crystal. Spectrochim Acta Part A Mol Biomol Spectrosc 150:765–771
Anjalin FM, Kanagathara N, Marchewka MK, Mohankumar V (2019) Crystal structure, Hirshfeld surface analysis and vibrational spectral studies on P-nitroanilinium p-toluene sulphonate single crystal. J Mol Struct 1183:78–86
Mahadevan M, Anandan P, Ramachandran K, Aravindhan M, Hayakawa Y (2014) Studies on the growth aspects and characterization of sodium para-nitro phenolate single crystals for nonlinear optical applications. Optik 125:5515–5518
Johnson J, Sreenivasan R, Sivavishnu D (2018) Process development and characterization of centrosymmetric semiorganic nonlinear optical crystal: 4-dimethylaminopyridine potassium chloride. Phys B Phys Condens Matter 538:199–206
Chinnasami S, Manikandan M, Chandran S, Paulraj R, Ramasamy P (2018) Growth, Hirshfeld surfaces, spectral, quantum chemical calculations, photoconductivity and chemical etching analyses of nonlinear optical p-toluidine p-toluenesulfonate single crystal. Spectrochim Acta Part A Mol Biomol Spectrosc 206:340–349
Shanlini S, Kirupavathy SS, Jerusha E (2019) Growth and characterisation of dimethylamino pyridinium dsp-bromo-chlorophenolate mixed crystal: a new organic NLO material for terahertz applications. J Mol Struct 1178:126–134
Panchapakesan Satchidhanandham, Subramani Karthick, Srinivasan Brahadeeswaran (2018) Growth, spectral, thermal, electrical, mechanical and nonlinear optical properties of organic single crystal 4–Amino–(1–methylphenyl) pyridinium bromide. Optik 157:774–786
Krishna Kumar V, Guru Prasad L, Nagalakshmi R (2009) Synthesis, growth, spectral, optical, thermal, and non-linear optical applications of an inorganic single crystal: potassium dihydrogen orthophosphate-sodium chloride single crystal. Eur Phys J Appl Phys 48(2009):20403–20409
Tauc J, Grigorovici R, Vancu A (1966) Optical properties and electronic structure of amorphous germanium. Phys Status Solidi 15:627
Chandra CS, Nagaraju D, ThirumalRao T, Raja Shekar PV, Gopi Krishna N (2013) Growth, dislocations and hardness studies of a nonlinear optical crystal-glycinium oxalate. Res J Phys Sci 1(8):1–5
Nageshwari M, Jayaprakash P, Kumari CR, Vinitha G, Caroline ML (2017) Growth, spectral, linear and nonlinear optical characteristics of an efficient semiorganic acentric crystal: L-valinium L-valine chloride. Phys B 511:1–9
Thomas P, Junjuri R, Joy N, Slemer M, Gundawar MK, Philip R, Al-Shamery K, Joseph GP (2019) Influence of MnCl2 on the properties of an amino acid complex single crystal-l-arginine perchlorate (LAPCl) for optical limiter applications. J Mater Sci Mater Electron 30:8407–8421
Parthasarathy M, Krishnan RG (2013) Growth, photoluminescence, thermal and mechanical behaviour of Ethyltriphenylphosphonium bromide dihydrate crystal. Opt Mater 35:2056–2061
Skoog DA (1971) Principles of instrumental analysis, 1st edn. Holt, Rinehart and Winston, New York
Bincy IP, Srinivasan T, Jaisankar SN, Ramkumar V (2019) Structure, growth and characterization of a new naphthalene family crystal for fluorescence and third order nonlinear optical applications. Solid State Sci 89:85–92
Mohan MK, Ponnusamy S, Muthamizhchelvan C (2017) Influence of ammonium dihydrogen phosphate (ADP) and L-tartaric acid on nonlinear optical triglycine sulphate (TGS) single crystals. Opt Laser Technol 97:321–326
Subhadra KG, Balaiah E, Sirdeshmukh DB (2002) Systematic hardness measurements on CsClx Br(1−x) and NH4Cl x Br(1−x) mixed crystals. Bull Mater Sci 25:31–35
Mukerji S, Kar T (1999) Vicker’s microhardness studies of l-arginine hydrobromide monohydrate crystals (LAHBr). Cryst Res Technol 34:1323–1332
Joshi MJ, Shah SS (1984) On the microhardness of some organic molecular solids. Cryst Res Technol 19:1107–1111
Vesta C, Uthrakumar R, Raj CJ, Varjula AJ, Linet JM, Das SJ (2007) Growth, Structural and microhardness studies on new semiorganic single crystals of calcium para nitrophenolate dihydrate. J Mater Sci Technol 23(6):855–859
Chacko E, Linet JM, Priya S, Vesta C, Boaz BM, Das SJ (2006) Growth and microhardness studies of mixed crystals of (NH4)2SbF5-K2SbF5. Indian J Pure Appl Phys 44:260–263
Sangwal K, Hordyjewicz M, Surowska BJ (2002) Microindentation hardness of SrLaAlO4 and SrLaGaO4 single crystals. J Optoelectron Adv Mater 4:875–882
Sangwal K (2000) On the reverse indentation size effect and microhardness measurement of solids. Mater Chem Phys 63:145–152
Shaw MC (1973) The fundamental basis of the hardness test. In: Westbrook JH, Conrad H (eds) The science of hardness testing and its research applications. American Society for Metals, Cleveland
Oliver WC, Hutchings R, Pethica JB (1986) In microindentation techniques in materials science and engineering. American Society for Testing and Materials (ASTM STP 889), Philadelphia, 90–108
Onitsch EM (1956) The present status of testing the hardness of materials. Mikroskopie 95:12–14
Hanneman M (1941) Indentation size effect and microhardness study. Metall Manch 23:135–139
Muthuraja A, Kalainathan S (2017) Study on growth, structural, optical, thermal and mechanical properties of organic single crystal ethyl p-amino benzoate (EPAB) grown using vertical Bridgman technique. J Cryst Growth 459:31–37
Ramteke SP, Anis M, Baig MI, Pahurkar VG, Muley GG (2017) Optical and electrical analysis of Cu2+ ion doped zinc thiourea chloride (ZTC) crystal: An outstanding 30 × 24 × 04 mm3 bulk monocrystal grown from pH controlled aqueous solution. Optik 137:31–36
Azhar SM, Anis M, Hussaini SS, Kalainathan S, Shirsat MD, Rabbani G (2017) Doping effect of l-cystine on structural, UV–visible, SHG efficiency, third order nonlinear optical, laser damage threshold and surface properties of cadmium thiourea acetate single crystal. Opt Laser Technol 87:11–16
Anis M, Muley GG (2016) Single crystal growth and enhancing effect of glycine on characteristic properties of bis-thiourea zinc acetate crystal. Phys Scr 91:85801–85808
Jayaprakash P, Sangeetha P, Mohamed MP, Vinitha G, Muthu S, Prakash M, Caroline ML (2017) Growth and characterization of dl-Mandelic acid (C6H5CH(OH)CO2H) single crystal for third-order nonlinear optical applications. J Mol Struct 1148:314–321
Kalaiselvi P, Raj SAC, Jagannathan K, Vijayan N, Bhagavannarayan G, Kalainathan S (2014) Solid state parameters, structure elucidation, High Resolution X-Ray Diffraction (HRXRD), phase matching, thermal and impedance analysis on l-Proline trichloroacetate (l-PTCA) NLO single crystals. Spectrochim Acta Part A. 132:726–732
Kityk IV, Marciniak B, Mefleh A (2001) Photoinduced second harmonic generation in molecular crystals caused by defects. J Phys D Appl Phys 34:1–4
Jayaprakash P, Sangeetha P, Kumari CR, Baskaran I, Caroline ML (2017) Growth and characterization of l-asparagine monohydrate admixtured dl-mandelic acid nonlinear optical single crystal. J Mater Sci Mater Electron 28:18787–18794
Urban BE, Lin J, Kumar O, Senthilkumar K, Fujita Y, Neogi A (2011) Optimization of nonlinear optical properties of ZnO micro and nanocrystals for biophotonics. Optic Mater Express 1:658–669
Vijayakumar P, Babu GA, Ramasamy P (2012) Synthesis, crystal growth and characterization of nonlinear optical organic crystal: p-Toluidinium p-toluenesulphonate. Mater Res Bull 47:957–962
Suresh S, Devi SR, Sornamurthy BM, Arivanandhan M, Kumar RM (2019) Growth, structural and optical studies of a novel nonlinear optical material: p-Toluidinium L-Tartrate. Optik 185:651–656
Shanmugam G, Brahadeeswaran S (2012) Spectroscopic, thermal and mechanical studies on 4-methylanilinium p-toluenesulfonate—a new organic NLO single crystal. Spectrochim Acta Part A Mol Biomol Spectrosc 95:177–183
Sathya K, Dhamodharan P, Dhandapani M (2017) Computational, spectral and structural studies of a new non-linear optical crystal: 2-hydroxy pyridinium 3,5-dinitrobenzoate. J Mol Struct 1130:414–424
Rathika A, Raman RG (2014) Investigations on growth and characterisation of 2-hydroxyanilinium 3,5-dinitrobenzoate single crystal. Mater Res Innov. https://doi.org/10.1179/1433075X14Y.0000000239
Sheik-Bahae M, Said AA, Wei T, Hagan DJ, Van Styrland EW (1990) Sensitive measurement of optical nonlinearities using a single beam. IEEE J Quantum Electron 26:760–769
Pramodini S, Poornesh P, Sudhakar YN, SelvaKumar M (2013) χ(3) and optical power limiting measurements of Polyaniline and its derivative Poly (o-toluidine) under CW regime. Opt Commun 293:125–132
Nagaraja KK, Pramodini S, Kumar AS, Nagaraja HS, Poornesh P, Kekuda D (2013) Third-order nonlinear optical properties of Mn doped ZnO thin films under cw laser illumination. Opt Mater 35:431–439
Sutherland RL (1996) Handbook of nonlinear optics. Marcel Dekker Inc., New York
John NL, Abraham S, Sajan D, Philip R, Joy N, Chitra R (2020) Molecular structure, NLO properties and vibrational analysis of l-Histidine tetra fluro borate by experimental and computational spectroscopic techniques. Spectrochim Acta Part A Mol Biomol Spectrosc 226:117615
CHEMCRAFT: Available online. http://www.chemcraftprog.com
Thanikaivelan P, Subramanian V, Rao JR, Nair BU (2000) Application of quantum chemical descriptor in quantitative structure activity and structure property relationship. Chem Phys Lett 323:59–70
Munsif S, Khan S, Ali A, Gilani MA, Iqbal J, Ludwig R, Ayub K (2018) Remarkable nonlinear optical response of alkali metal doped aluminum phosphide and boron phosphide nanoclusters. J Mol Liq 271:51–64
Khattak J, Ludwig R, Ayub K (2017) NANO-CAGES Phosphides or nitrides for better NLO properties? A detailed comparative study of alkali metal doped nano-cages. J Maerresbull 92:113–122
Maria J, Iqbal K (2016) Ayub, Theoretical study of the non-linear optical properties of alkali metal (Li, Na, K) doped aluminum nitride nanocages. RSC Adv 6:94228–94235
Acknowledgements
The authors sincerely thank to Prof. P.K Das, Department of Inorganic chemistry, IISc-Bangalore for extending help to carry out SHG measurement, VIT-Chennai for micro hardness analysis, Saran Raj, Sacred Heart College- Tirupattur for dielectric studies, Priya Dominic and Reji Philip, RRI-Bangalore for providing Z-Scan studies and Nirmalagiri College-Kerala and SAT campus, Kannur University-Kerala for providing various characteristic studies upon the synthesized material.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sreedharan, R., Ravi, S., Raghi, K.R. et al. Growth, linear- nonlinear optical studies and quantum chemistry formalism on an organic NLO crystal for opto-electronic applications: experimental and theoretical approach. SN Appl. Sci. 2, 578 (2020). https://doi.org/10.1007/s42452-020-2360-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2360-9