Abstract
In recent trends, monitoring and control of air quality have arisen, because there are many organic and inorganic pollutants that harm our air quality, formaldehyde is one of those substances. Therefore, it looks essential to monitor and control the exposure of formaldehyde in living environments. In this paper, we examine the reactivities of pure and doped (boron and nitrogen) single-walled carbon nanotube (3, 3) armchair with formaldehyde (CHOH) molecule via density functional theory (DFT) using Beck three-parameter, Lee–Yang–Parr method and 6-31(d, p) at room temperature. Through DFT we performed the molecular electrostatic potential, NBO analysis, HUMO–LUMO for calculating the electronic properties of the system considered. Based on which nitrogen-doped SWCNT (3, 3) shown high sensitivity to formaldehyde molecule compare to pure—SWCNT (3, 3) and boron-doped SWCNT (3, 3). N-doped SWCNTs are predictable to be a potential candidate for sensing the presence of formaldehyde.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent times, due to increasing commercial and transportation activities, the quality of air around us decrease significantly. Therefore, to improve the air quality necessary measures should be taken to overcome air pollution. It is necessary to monitor the amount of Formaldehyde (HCOH), an organic pollutant present in the air. It has been observed that the presence of HCHO in air shows the adverse effects on different parts of human beings such as sensory organs that are bare or come in direct contact with the air causing problems such as coughing, gasp, vomiting, and skin irritations are more prominent [1, 2]. Besides these, the momentary health problems of HCOH exposure are known but the long term health effects of HCOH exposure are still part of modern research. Some researches in the past indicate that the HCOH is human carcinogen under a long term exposure and develops certain kind of cancer [3, 4]. Therefore, monitoring and control of HCOH emissions in the living environment are of special interest.
To detect the presence of HCHO in the air sensors based on oxide thin films has been developed which exhibits sensitivities of 10 to 40 ppm [5,6,7]. However, the operating temperature of these thin-film sensors is in the range of 200–400 °C [8].There is a need to decrease the operating temperature so that sensors can operate at room temperature as well as they can maintain their sensitivity too. In this regard, nanoparticles materials like CNTs [9] and inorganic nanowires [10] are used in chemical sensors. Single-walled carbon nanotubes have remarkable, structural and electrical properties. They have various applications in nanoelectronics fields due to their prominent flexibility, unique electronic properties, and large surface area [11, 12]. These nanomaterials have the good capability as environmental sensors, monitoring, and control of exposure also detecting and removing several pollutants like organic chemicals and dioxin from the air [13]. Sensing of fragrant compounds like benzene, xylenes, natural organic materials, and poly aromatics on single-walled carbon nanotubes have been studied [14]. Single-walled CNTs have shows great sensitivity regarding molecules like NO, NOx and NH3 [15,16,17]. However, pure single-walled CNTs may not sense some highly toxic compounds like Formaldehyde. Functionalization of single-walled CNT or doping of atoms like oxygen, nitrogen, boron, etc. further increases their sensing and catalysis properties [18, 19]. For the detection of highly toxic gas, less work has been done including a theoretical study to develop CNT based nanosensors. Wang et al. investigation indicating the suitability of Al as a dopant in SWCNTs for sensing CO. [20] Zhang et al. and Wang et al. investigated chemical sensing of HCN or HCOH with boron-doped (B-doped) SWCNTs (8, 0) by using density functional theory calculations. Compared with the pure SWCNTs, B-doped SWCNTs presents a high response to HCN or HCOH [21, 22].Therefore in the present study of work we use pure and B- and N-doped SWCNTs as a chemical sensor for HCOH and aim to improve the sensitivity of pure single-walled carbon nanotubes by doping with boron and nitrogen.
2 Materials and methods
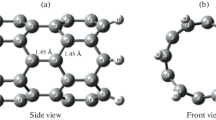
The density functional theory (DFT) method is the standard model for many computational chemistry software systems. All geometries have been fully optimized at the Density functional theory with B3LYP/6-31G (d, p) level with the help of the Gaussian 09 suite of programs at temperature 298.15 K [23]. We first generate (3, 3) armchair single-walled carbon nanotube (SWCNT) model by using nanotubes structure generator (TubeGen 3.4) [24]. In Fig. 1b, where the C atom in position (1) will replace by a nitrogen and boron atoms respectively to generate nitrogen-doped SWCNT and boron-doped SWCNT models, also the open ends of the nanotubes are bonded with hydrogen atoms to refrain from boundary effects. We performed optimizations and calculations of pure-SWCNTs, nitrogen and boron- doped SWCNTs with and without formaldehyde. To study the possible adsorption between Pure- and doped- SWCNT with Formaldehyde.
3 Results and discussion
3.1 Molecular electrostatic potential (MEP) analysis
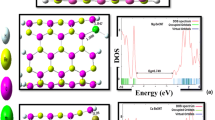
The molecular electrostatic potential (MEP) is related to the electron density and it is performed to predict reactive sites of the investigated molecule for electrophilic or nucleophilic attacks. [25] The MEPs of molecule Formaldehyde, pure-SWCNT (3, 3)/doped-SWCNT (3, 3) and complexes were prevailed by theoretical studies with the help of B3LYP-6-31G (d, p) method and the charge distribution were calculated by molecular electrostatic potential (MEP) shown in Fig. 2. It was found from MEP maps of HCOH that O atom shows highest electron density with red color and H has less electron density with green color as compared to O atom. [26] The O atom in formaldehyde molecule O atom is shown reactive sites and provides the possibility for a Formaldehyde molecule to approach the Pure and Doped carbon nanotube. Therefore, the Formaldehyde HCOH molecule can approach the Pure- SWCNT (3, 3) and Doped (B- and N-) CNT (3, 3) with different orientations.
3.2 Natural Bond Orbitals (NBO) Analysis
Natural Bond Orbitals study was performed via hybrid function B3LYP and basis set 6-31G (d, p) level. NBO analysis is executed to determine inter- and intra-molecular bonding and interaction between the donor and acceptor molecular system [27]. In NBO analysis the electron delocalization from Lewis valence orbitals (donor) to non-Lewis (acceptor) orbitals describes an extended molecular orbital that increase the stability of the system and explains electron transfer process between complexes [28]. The stabilization energy E(2) of interaction associated with the delocalization between i → j is calculated as
where qi and F (i, j) are represent the occupancy of donor orbital and off—diagonal Fock matrix element respectively, and εi and εj are energies of orbital [29].
The NBO analysis for complex 1, complex 2, and complex 3 has been implemented by B3LYP-6-31G (d, p) and the analysis is reported in Table 1. The O atom (LPs) of Formaldehyde molecule interact with the anti-bonding (BD*) orbital σ*(C37–H49) and σ*(C46–H47) of the Pure-CNT (3, 3) in the complex1, σ*(C36–H48) and σ*(C45–H46) of the B-CNT (3, 3) nanotube in the complex 2 and π*(C26–C27) and π*(C37–C38) of the N-CNT (3, 3) nanotube in the complex 3. Charge transfer of electron occurs as LP(1)O62 → σ*(C37–H49), LP (1)O62 → σ*(C46–H47) in complex 1, LP (1)O60 → σ*(C36–H48), LP(2)O60 → σ*(C45–H46) in complex 2, LP(3)O62 → π*(C37–C38), LP(2)O62 → π*(C26–C27) in complex 3 with hyper conjugative energies ∆E(2); 0.21, 0.28, 0.26, 1.15,2.60 and 0.52 kilocalorie per mole respectively. Therefore NBO analysis showed the strong interaction and charge transfer between the HCOH and boron and nitrogen-doped SWCNTs.
3.3 Electronic Properties
To analyze the adsorption between a Formaldehyde (HCOH) and Pure-SWCNT (3, 3) or B-doped SWCNT and N-doped SWCNT, we observe their adsorption energy ∆Eads, defined as
where E complex, ESD and EF are the optimized energies of the complexes (1, 2 and 3), SWCNTs (pure or doped) and Formaldehyde structure respectively, the calculated adsorption energies are shown in Table 2. Accordingly the adsorption energy value was found to be 0.98156 eV, 0.99785 eV and − 1.3319 eV of complex 1, complex 2 and complex 3 respectively. In complex 1 and 2, a small adsorption energy ∆Ead value indicates that Formaldehyde (HCOH) molecule undergoes physical adsorption on pure and B-doped SWCNT (3, 3) nanotube. The physical adsorption occurs due to weak van der Waals interaction between the pure or B-doped SWCNT (3, 3) and Formaldehyde molecule. The adsorption energy ∆Ead of complex 3 (Formaldehyde (HCOH)-nitrogen-doped SWCNT) has a negative value of about − 1.3319; this means adsorption takes place between them (attraction) and the exothermic reaction performed thermodynamically [30]. The interaction between the complexes can be best explained in terms of HOMO and LUMO energies. HOMO can donate electrons, while LUMO has a similar adverse effect. If a molecule has high HOMO energy then it will be more reactive (unstable) and vice versa [31].
HOMO and LUMO of SWCNTs/B- and N-doped SWCNTs before and after interaction with formaldehyde are given in Table 2 and shown in Fig. 3. On the basis of computational results, HOMO energy of N-doped SWCNTs decrease of 0.58 eV, 0.17 eV in B- doped SWCNTs, 0.02 in SWCNTs on sensing Formaldehyde. The weak interaction is observed in SWCNTs/B-doped SWCNTs compared to N-doped SWCNTs (see in Table 2). The corresponding HOMO energy of N-doped SWCNTs decrease on sensing, which indicates electron transfers from formaldehyde molecule to N-doped SWCNTs. The energy band gap (Eg) between the HOMOs and LUMOs calculated using the B3LYP method and 6-31G (d, p) basis set. The energy band gap Eg of pure- SWCNT (3, 3), B-doped SWCNT (3, 3) and N-doped CNT (3, 3) are 1.93 eV, 1.86 eV, and 1.61 eV respectively, after the adsorption of formaldehyde molecule in complex 1 (1.94 eV) and complex 2 (1.90 eV), we do not observe much changes in the energy gaps of complex 1 and complex 2, which indicates in complex 1 and complex 2, no adsorption takes place or adsorptions do not change the electronic properties of the pure- SWCNT (3, 3) and B- doped SWCNT (3, 3), but in complex 3 (N-doped SWCNT- HCOH), we observe the remarkable change in the energy gap (1.24 eV).The energy gap (Eg) value of N-doped CNT (3, 3) decreases after adsorption of HCOH molecule as reported in Table 2. This indicates the strong interaction between the N-doped CNT (3, 3) and Formaldehyde (HCOH).
A molecular descriptors of the Formaldehyde (HCOH), Pure- and doped SWCNT (3, 3) armchair nanotube and complex 1, 2 and 3 involve chemical softness (S), ionization potential (I), electron affinity (A), electronic chemical potential (μ), electrophilicity (ω), and global hardness (η) and electronegativity (χ) we can calculate above.
Parameters with the help of following equations:
as analyzed in Table 2. The electronic chemical potential (μ) and global hardness (η) values decreased after the adsorption of HCOH on the nanotube in complex 3 which indicates the high chemical activity and low chemical stability [32]. Comparatives analysis of ∆Eads, Eg, and HOMO–LUMO energies in complexes reveals that nitrogen-doped SWCNTs have a greater response to the detection of formaldehyde.
4 Conclusion
The computational results of NBO analysis and electronic properties show that nitrogen-doped SWCNTs have a remarkable change in energy gap from 1.61 to 1.24 eV after the adsorption of HCOH reveals that nitrogen-doped SWCNT has a greater response to HCOH, also the complex 3 have lower adsorption energy − 1.3319 kcal/mol indicates strong interaction between the HCOH and nitrogen-doped CNT. In Summary, DFT calculation results show that nitrogen-doped single-walled carbon nanotube is a promising candidate for sensing formaldehyde (HCOH) molecules.
References
Grammer LC, Harris KE, Cugell DW, Patterson R (1993) Evaluation of a worker with possible formaldehyde-induced asthma. J Allergy Clin 92(29):33
Latorre N, Silvestre JF, Monteagudo AF (2011) Allergic contact dermatitis caused by formaldehyde and formaldehyde releasers. Actas Dermo-Sifiliograficas 102:86–97
Beane Freeman L, Blair A, Lubin JH et al (2009) Mortality from lymphohe matopoietic malignancies among workers in formaldehyde industries: The National Cancer Institute Cohort. J Natl Cancer Inst 101(10):751–761
Registry of Toxic Effects of Chemistry Substances (1976) National Institute of Occupational Safety and Health, Washington, DC
Dirken JA, Duval K (2001) NiO thin-film formaldehyde gas sensor. Sensors Actuators B 80:106
Chen T, Liu QJ, Zhou ZL, Wang YD (2008) The fabrication and gas-sensing characteristics of the formaldehyde gas sensors with high sensitivity. Sensors Actuators B 131:301
Lee CY, Chiang CM, Wang YH, Ma RH (2007) The fabrication and gas-sensing characteristics of the formaldehyde gas sensors with high sensitivity. Sensors Actuators B 122:503
Wang J, Liu L, Cong SY, Qi JQ, Xu BK (2008) An enrichment method to detect low concentration formaldehyde. Sensors Actuators B 134:1010
Kauffman DR, Star A (2008) Carbon nanotube gas and vapor sensors. Angew Chem Int Edn 47:6550
Meyyappan M, Sunkara M (2009) Inorganic nanowires: applications, properties and characterization. CRC Press, Boca Raton, FL
Bandaru PR (2007) Electrical properties and applications of carbon nanotube structures. J Nanosci Nanotechnol 7:1239–1267
Maniwa Y, Fujiwara R, Kira H, Tou H, Nishibori E, Takata M, Sakata M, Fujiwara A, Zhao X, Iijima S, Ando Y (2001) Multiwalled carbon nanotubes grown in hydrogen atmosphere: an x-ray diffraction study. Phys Rev B 64:073105
Fam DWH, Palaniappan A, Tok AIY, Liedberg B, Moochhala SM (2011) A review on technological aspects influencing commercialization of carbon nanotube sensors. Sens Actuators B Chem 157:1–7
Ching-J Monica C, Shih MW, Tsai HJ (2010) Adsorption of nonpolar benzene derivatives on single-walled carbon nanotubes. Appl Surf Sci 256:6035–6039
Feng X, Irle S, Witek H, Morokuma K, Vidic R, Borguet E (2005) Sensitivity of ammonia interaction with single-walled carbon nanotube bundles to the presence of defect sites and functionalities. J Am Chem Soc 127:10533–10538
Li J, Lu YJ, Ye Q, Cinke M, Han J, Meyyappan M (2003) Carbon nanotube sensors for gas and organic vapor detection. Nano Lett 3(7):929–933
Beheshtian J, Kamfiroozi M, Bagheri Z, Ahmadi A (2011) Computational study of CO and NO adsorption on magnesium oxide nanotubes. Physica E 44(3):546–549
Dwivedi N, Srivastava D, Shukla RK, Srivastava A (2019) Spectroscopic study of large-scale synthesized, nitrogen-doped carbon nanotubes using spray pyrolysis technique. Mater Today Proc 12:590–595
Pylypenko S, Borisevich A, More KL, Corpuz AR, Holme T, Dameron AA, Olson TS, Dinh HN, Gennett T, O’Hayre R (2013) Nitrogen: unraveling the secret to stable carbon-supported Pt-alloy electrocatalysts. Energy Environ Sci 6:2957–2964
Wang R, Zhang D, Sun W, Han Z, Liu C (2007) A novel aluminum-doped carbon nanotubes sensor for carbon monoxide. J Mol Struct 806(1–3):93–97
Zhang YM, Zhang DJ, Liu CB (2006) Novel chemical sensor for cyanides: boron-doped carbon nanotubes. J Phys Chem B 110:4671
Wang R, Zhang D, Zhang Y, Liu C (2006) Boron-doped carbon nanotubes serving as a novel chemical sensor for formaldehyde. J Phys Chem B 110(37):18267–18271
Gaussian 09, Revision D.01, Frisch M J, Trucks GW, Schlegel HB, Scuseria GE et al. (2009) Gaussian, Inc., Wallingford CT
TubeGen 3.4 (web-interface, http://turin.nss.udel.edu/research/tubegenonline.html), Frey JT, Doren DJ (2011) University of Delaware, Newark DE
Luque FJ, Lopez JM, Orozco M (2000) Perspective on “Electrostatic interactions of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects. Theor Chem Acc 103(3):343–345
Moro S, Bacilieri M, Ferrari C, Spalutto G (2005) Autocorrelation of molecular electrostatic potential surface properties combined with partial least squares analysis as alternative attractive tool to generate ligand-based 3D-QSARs. Curr Drug Discov Technol 2(1):13–21
Glendening ED, Landis CR, Weinhold F (2012) Natural bond orbital methods, Wiley Interdiscip. Rev Comput Mol Sci 2:1–42
Sheikhi M, Shahab S, Filippovich L, Yahyaei H, Dikusar E, Khaleghian M (2018) New derivatives of (E, E)-azomethines: design, quantum chemical modeling, spectroscopic (FT-IR, UV/Vis, polarization) studies, synthesis and their applications: Experimental and theoretical investigations. J Mol Struct 1152:368–385
Weinhold F, Landis C (2005) Valency and bonding: a natural bond orbital donor—acceptor perspective. Cambridge University Press, Cambridge
Sheikhi M, Shahab S, Khaleghian M, Kumar R (2018) Interaction between new anti-cancer drug syndros and CNT(6,6-6) Nanotube for medical applications: geometry optimization, molecular structure, spectroscopic (NMR, UV/Vis, Excited state), FMO, MEP and HOMO-LUMO investigation. Appl Surf Sci 434:504–513
Ullah H, Haq Ali H, Bilal SS, Ayub K (2013) DFT study of polyaniline NH3, CO2, and CO gas sensors: comparison with recent experimental data. J Phys Chem C 117:23701–23711
Sheikhi M, Shahab S, Alnajjar R, Ahmadianarog M, Kaviani S (2019) Investigation of adsorption tyrphostin AG528 anticancer drug upon the CNT (6, 6-6) nanotube: a DFT study. Curr Mol Med 19(2):91–104
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dwivedi, N., Shukla, R.K. Theoretical study of pure/doped (nitrogen and boron) carbon nanotubes for chemical sensing of formaldehyde. SN Appl. Sci. 2, 262 (2020). https://doi.org/10.1007/s42452-020-2055-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2055-2