Abstract
In the present paper, films and nanofibers of zein and zein/tryptophan were produced by casting and eletrospinning.The films and nanofibers were characterized by scanning electron microscopy (SEM), thermogravimetric analysis (TGA), Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) and controlled release and digestibility tests. In vitro releasing of tryptophan was evaluated using a spectrophotometer UV–Vis. Interactions between tryptophan and zein were confirmed by thermal (TGA) and spectral (FTIR) analyses. SEM micrographs provided evidence of a smooth and homogeneous structure for films and nanofibers without and with tryptophan. FTIR spectroscopy indicated second-degree intermolecular interactions between amide groups of zein and tryptophan. X-ray analysis indicated the formation of crystals that did not interfere with the crystallization of amino acid. By in vitro release experiments, it was possible to observe that the tryptophan releasing follows nearly Korsmeyer–Peppas model for the nanofibers and Peppas–Sahlin model for films, indicating the releasing process of the nanofibers are by diffusion of the amino acid and, for films, by relaxion of polymeric chain. Finally, in vitro digestibility a considerable amount of tryptophan was observed, suggesting more access of fishes for tryptophan.
Similar content being viewed by others
1 Introduction
Fish farming comprises a constantly expanding aquaculture sector in Brazil [1]. Aquaculture was the most prominent meat production sector between 2004 and 2014, with an average growth of 8%, against 4.1% for poultry farming, 5.1% for cattle raising and 2.9% for pig farming. Tilapia, the main grown aquaculture species in the country, presented an average production increase of 14.2% per year in the same period [2]. Its meat characteristics and the ease of animal breeding, are increasingly attracting the market. However, the technological package for tropical fishes commercial production still needs adjustments, such as nutritional aspects of aquafeed [3]. Diets with insufficient protein levels can slow fish growth, compromise food efficiency, or mobilize protein from some tissues to maintain other vital functions [4]. Thereby, several studies [5, 6] search to develop more efficient systems for making essential amino acids available in the diet of tropical fishes.
To meet the fish production in the country, considering the system of cultivation in cages and ponds, it is important that the animals receive a diet capable of meeting the required nutritional requirements. The essential amino acid tryptophan is present in the composition of feed used in the manufacture of feed [7]. Knowledge about the stability of feed components should also be considered. When in contact with water, some of these substances may be lost to the environment [8], making it impossible to ingest them. To this end, it is interesting to develop strategies capable of preventing or minimizing these losses arising from the lack of stability of the substances, ensuring that more nutrients are accessible to animals. Nutrient encapsulation in polymeric nanofibers is an effective alternative that can ensure the best use of these substances by fish.
The high surface area of nanofibers, when compared to volume and porosity, are properties that make them considerably attractive, especially due to their production from the electrospinning technique, where polymers, biopolymers, metals, and metal oxides can be employed in the process [9,10,11,12,13,14]. These nanostructures have varied applications, and, among them, it is possible to mention the controlled release systems where nanofibers can be used to deliver drugs, nutrients, or fertilizers. Although entrapment of nutraceuticals can be readily achieved by other methods, the repertoire of studies to encapsulate essential amino acids into nanostructured fish feed is very limited.
The development of a slow-release system can reduce the diffusion of amino acids to the aquatic environment and optimize the amount of them available to tropical fish. In this way, it will contribute significantly to the reduction of costs in the formulation of aquafeed. Also, this study aims to investigate the effect of the high surface area of biopolymeric nanofibers on the release kinetics of amino acids in zein matrices. In this context, the present work proposes to develop and characterize hydrophobic nanofibers and encapsulated zein films with the tryptophan amino acid, considered indispensable in the fish nutrition process. In addition to the characterization techniques to be employed, the nanostructures will be evaluated for the in vitro release profile of the amino acid in solutions that simulate the aquatic environment and the digestive system of tropical fish. This paper analyzes the efficiency of the encapsulation process, which will determine the production viability of the more efficient nanostructured fish feed.
2 Experimental section
2.1 Materials
Zein was obtained from Sigma-Aldrich (USA), and the tryptophan from Synth (Brazil). Ethanol was purchased from Synth (Brazil) and deionized water was used as a solvent. Sodium bicarbonate (NaHCO3), sodium hydroxide (NaOH) and chloridric acid (HCl) and were purchased from Exodo (Brazil).
2.2 Solution, film, and nanofiber production
The solution of zein 30% (w/v) was dissolved in ethanol: distilled water at a proportion of 80:20 (v/v) under continuous stirring [15], for 2 h at room temperature, until complete dissolution of biopolymer. Tryptophan was added in the biopolymer solutions at the concentration of 0 and 30% (w/w), based on the weight of the zein. The solution of zein-tryptophan was left under stirring for 1 h at room temperature.
The films were obtained by casting technique using zein and zein—tryptophan solutions. The mats of zein were obtained by electrospinning, which set up consisted of a high voltage source (INSTRUM, model HIPOT 60 kV, 5 mA), a syringe flow pump (NEW ERA PUMP SYSTEMS, model Syringe Pump AL1000) and a plastic syringe of the volume of 5 mL and 0.7 mm of diameter. The experiment was performed by 27 kV of voltage source with a pump flux of 0.08 μL min−1, with a distance between the syringe and the collector of 7.5 cm. The nanofibers were collected on sheet metal, wrapped with aluminum foil, generating non-woven nanofiber mats. The contact between the high voltage source and needle surface and the contact between the high voltage source and the sheet metal was performed through electrodes. The experimental apparatus was isolated inside a glass front-opening wooden box to maintain moisture control throughout the process. The experiment was conducted at room temperature. The nanofiber mats were then collected by peeling off and stored in a desiccator.
2.3 Film and nanofiber characterization
Film surface and nanofiber morphology were observed using LEO EVO 40 XVP (Carl Zeiss) scanning electron microscope (SEM) coated with gold by sputtering (Balzers, SCD 050). The diameter of the nanofiber was measured with the aid of an image analysis software (Image J, National Institutes of Health, USA). For each experiment, the average nanofiber diameter and distribution were determined from approximately 100 measurements randomly taken from representative nanofiber morphology. The fracture surfaces (transversal area) obtained after immersing biopolymer film in liquid air (fragile fracture) were observed using LEO EVO 40 XVP (Carl Zeiss) scanning electron microscope (SEM) coated with gold by sputtering (Balzers, SCD 050) [16].
Fourier transform infrared spectroscopy (FTIR) was performed by Nicolet 470 Nexus FTIR spectrometer. The FTIR spectrometer was purged continuously with nitrogen. A total of 64 scans were considered with a resolution of 2 cm−1. The infrared spectra were recorded in transmission mode on thick blow spinning samples deposited on a silicon wafer.
X-ray diffraction (XRD) patterns were generated from non-woven fibrous mats using a Shimadzu XRD-6000 Diffractometer with a Ni filtered CuKα radiation (1.54 Å) at 50 kV and 20 mA. Scans were carried out from 5° to 30° (2θ) at a scan rate of 2°/min. The size of the crystallites of zein was measured by the Scherrer equation_ENREF_15.
Thermal analysis was conducted by the thermogravimetric analyzer (TGA) in a Q500 TA Instruments under the nitrogen atmosphere, at a flow rate of 20 mL.min−1. Samples were scanned from room temperature to 600 °C at a scanning rate of 10 °C/min using platinum crucibles.
2.4 Assessment of tryptophan in vitro release
Approximately 25 mg of zein/tryptophan films and nanofibers were allocated into a falcon tube with 50 ml of the aqueous solution with 40 mg/L of NaHCO3 at the controlled temperature and pH’s (at 25 °C and pH = 5.5, 25 °C and pH = 8.5 and 35 °C, and pH = 5.5). The amount of tryptophan was assessed by intermittently aliquot sampling (around 180 µL), taken off from falcon tube. The aliquots were submitted to a UV–visible spectrophotometer (Shimadzu, model UV-2601) around a wavelength of 280 nm. For all conditions, the falcon tubes were kept on shaking at 120 rpm during the experiment. To analyze in vitro release kinetics of the nanofibers, a Korsmeyer–Peppas (Eq. 1) and Korsmeyer–Peppas with Tlag (Eq. 2) models were used:
where Mt/M∞ is a fraction of tryptophan at time t, k is the release rate constant and n is the release exponent. The n value is used to characterize different releases for cylindrical shaped matrices.
Peppas–Sahlin (Eq. 3) and Peppas–Sahlin with Tlag (Eq. 4) models were also tested for the controlled release of films:
where k1 and k2 are constant and m is the Fickian diffusion exponent for a system of any geometric shape.
2.5 Tests of digestibility in vitro
In vitro digestibility tests were performed based on Wang et al. methodology [11]. First, 20 mg of the films and of the nanofibers of zein/tryptophan were submitted to fish stomach simulation, which were placed in 40 mL of gastric fluid simulation (5% w/v of pepsin in 0.15 M of NaCl). The gastric fluid solutions were acidified until the pHs reached 1.8, 2.5, and 3.5. The experiment was kept on shaking of 100 rpm, during 1 h in the dark (the flasks were covered with aluminum paper to prevent ambient light) and at room temperature.
Second, 20 mg of the films and of the nanofibers of zein/tryptophan were submitted to fish intestine simulation, which were placed in 40 ml of intestinal fluid simulation (1.5% w/v pancreatin, 0.5% w/v amylase, 0.3% w/v bile salts, and 0.15 M NaCl). The intestinal fluid solutions were acidified until reached the pHs of 4.5, 5.5 and 6.5. The experiment was kept on shaking of 30 rpm, during 5 h in the dark (the flasks were covered with aluminum paper to prevent ambient light) and at room temperature.
Released tryptophan in the solutions was quantified using a UV–Vis spectrophotometer.
3 Results and discussions
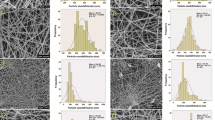
Figure 1 shows SEM images of the films and nanofibers of zein and zein/tryptophan (30% of amino acid). Zein and zein/tryptophan films have a homogeneous smooth surface (inset images a1 and b1) and have cross-section thicknesses of 0.48 mm for zein and 0.50 mm for zein/tryptophan. The heterogeneous morphology of the film surface, made by cryogenic fracture, for zein/tryptophan film could indicate a process of phase separation.
Electrospinning nanofibers with uniform morphology and narrow size distribution were produced, independent of the precursor solution. Both nanofibers have a smooth surface and no presence of tryptophan crystals. This result suggests that amino acid was dispersed homogeneously in the films and nanofibers. The distribution of diameters for the both nanofibers is seen in Fig. 1e. The average diameters ranged from 676 ± 116 nm for zein nanofibers to about 740 ± 117 nm for zein/tryptophan nanofibers. The increase in the average nanofiber diameter due to active agents addition is similar to results found in the literature [17,18,19] and can be interpreted as a success in the incorporation of an essential amino acid (tryptophan) in the zein matrix.
FTIR spectra of zein and zein/tryptophan nanofibers are shown in Fig. 2, from 4000 to 400 cm−1 region. The films spectra show similar bands found for nanofibers. The assigned peaks for zein spectra agree with the bands previously reported in the literature. Three characteristic peaks at 3307, 1650, 1538 and 1361 cm−1, for neat zein, correspond to the NH stretching, to amide I (C=O), amide II (N–H bending) and amide III (C–N), respectively [20]. For the sample with tryptophan the characteristic peaks are 3402, 3037, 1589, 1411, 1357 and 1059 cm−1, that correspond to N–H stretching in amines, C–H stretching in alkenes, N–H bending in amines, C–H bending in alkanes and C–N sketching in aryl amines [21].
By comparing the spectra, it can be concluded that the presence of tryptophan did not significantly affect the backbone structure of zein. However, modification in in the amide I and amide II are observed, which are related bands (1650 and 1538 cm−1) where a closer investigation reveals a zein/tryptophan interaction. Other studies report that shifts of amide I and II were associated with the occurrence of electrostatic interactions between proteins and other chemical components [19]. The relative intensity of amide I e amide II did not undergo a statistically significant change to zein/ tryptophan combination and can be numerically assessed as the amide I/amide II ratio which can be calculated as the absorbance values at 1650/1538 cm−1. This ratio was found to be 0.925 for the zein, 0.967 for the zein film, 0.988 for the zein/ tryptophan film, 0.948 for the zein nanofiber and 0.973 for the zein/tryptophan nanofiber. This result indicates that there was no chemical bond formation, only second-order intermolecular interactions between zein and tryptophan.
Figure 3 shows the X-ray diffraction patterns of the evaluated samples. As previously reported in the literature [22, 23], zein nanofiber has an amorphous peak around 2θ = 9º, and another wide peak around 2θ = 20º. The first peak, the shorter peaks, represented the intra-helix average backbone distance (α-helix structure of pure zein), while broader peaks are related intra-helix packing between the neighboring chains [3, 24, 25] the pure zein powder exhibits the same trend of structure, this is evidence that zein nanofiber did not change its structure.
Tryptophan powder has been reported to have crystalline peaks around the 2θ = 9.8°, 14.7°, 19.8°, 23.0°, 24.7°, 35.0° [26]. The current measurements reveal an increase in the crystallite size of tryptophan into zein nanofibers. These results also indicate that in the crystallization of zein/tryptophan nanofibers when produced by electrospinning, the nucleation and growth step is controlled by the interaction of biopolymer and amino acid in the blend. Although the crystalline percentage of the material was not influenced by the way zein and tryptophan were processed, as well as solvent extraction, it was possible to observe the formation of new crystalline structures not present in the other spectra in zein/tryptophan nanofibers when produced by electrospinning, possibly due to the rapid extraction of the solvent and the presence of the magnetic field that aligns the nanofibers towards them.
Thermogravimetric analysis (TGA) was used to determine onset degradation temperature (Tonset) for neat zein nanofibers and films and zein/tryptophan nanofibers as shown in Fig. 4. It is observed that the difference between the zein/tryptophan systems releasing is basically related to the thermal degradation of zein occurs in 2 stages. The first stage, at a temperature of 30–105 °C, indicates loss of water and low molecular mass (plasticizers and volatile compounds) [27, 28]. The second stage, at a temperature of 250–450 °C is related to protein degradation. The changes in the protein structure, provoked by the rupture of low energy intermolecular bonds, can promote a reduction in the thermal stability of the protein [29, 30].
Based on the values obtained after the controlled release test in water (Fig. 5), it was observed that the zein/tryptophan film under certain conditions did not release tryptophan in the first 15 min of the experiment. The zein/tryptophan nanofibers presented a high percentage of released tryptophan at the beginning of the experiment (burst release) but presented a lower percentage at the end of the experiment (slow release). Initial burst release followed by slower release mechanism were reported by literature [31] for biopolymeric nanostructure matrix. Regarding the evaluated parameters, the increase of the experiment temperature also increased the amount of tryptophan released, but it is not a statistically relevant amount. The same behavior was observed with the increase of pH in zein/tryptophan films. Regarding nanofibers, the same behavior was observed in relation to temperature, however, the increase in pH led to a slightly lower amount of tryptophan released, but it was not yet a relevant statistical difference. A higher release rate of tryptophan was observed in zein nanofibers compared to zein films. This higher release rate of the amino acid is due to the high surface area of the zein nanofibers.
Evaluating the proposed mathematical models and observing the constants present in Table 1, there is different mechanisms of tryptophan release for the films and nanofibers The film fits well with Peppas–Sahlin models and the nanofibers fit well with the Korsmeyer–Peppas models.
In the Korsmeyer–Peppas models, the value of n characterizes the release mechanism of tryptophan for cylindric samples 0.45 ≤ n corresponds to a Fickian diffusion mechanism, 0.45 < n < 0.89 to non-fickian transport, n = 0.89 to case II (relaxational) transport, and n > 0.89 to super case II transport [32, 33]. The results of the Table 1indicate that the mechanism of release of tryptophan molecules in nanofibers occurs strictly by Fickian diffusion governed by the difference in chemical potential between the inside of the zein nanofibers and the external aquatic environment. A diffusion mechanism has previously been reported from other groups working with zein nanofibers [20, 34]. Zein based matrices were previously reported to have release exponent less than 0.45 [35]. In this study, nanofibers had Fickian diffusion (n < 0.1), and zein films showed polymer relaxation release mechanism (k1 > > k2). Based on these results, it can be proposed that zein nanofibers, which have high surface area show Fickian diffusion mechanism for tryptophan, while in zein films polymer relaxation is the dominant release mechanism. However this model can only be applied under certain conditions such as zein matrix geometry is cylindrical (nanofibers), water and drug diffusion coefficients vary as functions of water concentration, biopolymer dissolution is incorporated, and change in zein matrix volume is considered according to Singhvi et al. [33].These results suggest that the tryptophan release from the zein nanofibers is through diffusion. Furthermore, it occurs due to the diffusion of the dissolution medium into the zein nanofibers, the dissolution medium solubilized the tryptophan and releases it slowly.
Fitting the release data of the tryptophan for the films, the Peppas–Sahlin constants (k1 and k2) may suggest the mechanism of release of tryptophan: if k1 > > k2 the release mechanism of the amino acid is controlled by the polymer relaxation. The fits of Peppas–Sahlin and Peppas–Sahlin with Tlag presented very small or negative k2 for films, indicating that this mechanism is controlled by relaxation of polymer chain. Finally, the release of tryptophan from zein matrices occurs by different mechanisms: in the case of nanofibers was observed Fickian diffusion and in the films was observed chain relaxation. This information on the release of tryptophan in aquatic environments with pH values of 5.5 and 8.5 and the temperatures of 25 and 35 °C is extremely important for tropical pisciculture, because these conditions are found in fish farming tanks in Brazil.
Table 2 shows the values of the in vitro digestibility test for the simulated fluid gastric and simulated fluid intestinal. The pH conditions adopted in the digestibility experiments are based on possible values found in the digestive system of tropical fish [36,37,38]. It can be seen that zein/tryptophan nanofibers had a slight increase in tryptophan digestion in both gastric and intestinal fluid digests in most cases, comparing the same pH conditions [39]. This can be explained by the increased surface area in the nanofibers, which speeds up the release process [6, 40]. It was not possible to observe a relationship between the pH change and the amount of tryptophan released through the static tests [41]. In most cases, there was no statistical difference with the pH variation, which suggests that pH variation does not interfere with the amount of tryptophan digested.
4 Conclusion
Tryptophan encapsulated in a zein matrix at the micro- and nano-level was successfullly obtained by film casting and electrospinning processes, respectively. Compared to casting films, nanoencapsulation by the electrospinning technique exhibited good morphology, highest surface area, and more homogeneous morphology. Electrospinning proved to have higher encapsulation efficacy than the film casting technology. The usage of zein as a carrier for tryptophan increases its release efficiency. In vitro simulated gastrointestinal stability studies showed that the release of encapsulated tryptophan from nanofibers is faster than from films, due to the larger surface area interacting with the release medium. Thus bioaccessibility of tryptophan is higher for electrospun nanofiber than zein films. The results of the current study confirm some miscibility of tryptophan and zein according to morphological, thermal, and spectroscopic used methods. Thus, electrospinning technology can be appropriated used to encapsulate the tryptophan in a zein matrix, providing a release with Korsmeyer–Peppas Kinect rates. The zein/tryptophan film demonstrated good performance in amino acid encapsulation and showed a good fit to the Peppas–Sahlin model. This study clearly indicated that zein films and zein nanofibers can be potentially used in the controlled delivering system of tryptophan, being a potential for the use of this material in fish farming. Thus, the nanometric sizeand processing conditions play an important role in the release and digestibility of tryptophan. Thus, tryptophan encapsulation in the zein matrix at the nanoscale achieved by electrospinning provides better bioaccessibility.
References
Roriz GD, de Delphinoa VCMK, Gardner IA, Picao GVS (2017) Characterization of tilapia farming in net cages at a tropical reservoir in Brazil. Aquac Rep 6:43–48. https://doi.org/10.1016/j.aqrep.2017.03.002
Schulter E, Vieira FJE (2018) Desenvolvimento e potencial da tilapicultura no Brasil. Revista de Economia e Agronegócio 16:177–201. https://doi.org/10.25070/rea.v16i2.554
Neo YP, Ray S, Jin J, Gizdavic-Nikolaidis M, Nieuwoudt MK, Liu D, Quek SY (2013) Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: a physicochemical study based on zein-gallic acid system. Food Chem 136:1013–1021. https://doi.org/10.1016/j.foodchem.2012.09.010
Carneiro WF, Castro TFD, Orlando TM, Meurer F, de Paula JDA, do Virote CRB, da Vianna CBAR, Murgas LDS (2020) Replacing fish meal by Chlorella sp. meal: effects on zebrafish growth, reproductive performance, biochemical parameters and digestive enzymes. Aquaculture 528:735612. https://doi.org/10.1016/j.aquaculture.2020.735612
Vera LM, Hamre K, Espe M, Hemre G-I, Skjærven K, Lock E-J, Prabhu AJ, Leeming D, Migaud H, Tocher DR, Taylor JF (2020) Higher dietary micronutrients are required to maintain optimal performance of Atlantic salmon (Salmo salar) fed a high plant material diet during the full production cycle. Aquaculture 528:735551. https://doi.org/10.1016/j.aquaculture.2020.735551
Li X, Liu Y, Yu Y, Chen W, Liu Y, Yu H (2019) Nanoformulations of quercetin and cellulose nanofibers as healthcare supplements with sustained antioxidant activity. Carbohydr Polym 207:160–168. https://doi.org/10.1016/j.carbpol.2018.11.084
Pezzato LE, Barros MM, Furuya WM (2009) Nutritive value of common feeds used in tropical fish diets. Revista Brasileira de Zootecnia-Braz J Anim Sci 38:43–51. https://doi.org/10.1590/S1516-35982009001300005
Barbosa ACA, de Sousa RV (2010) Effects of leaching in food for Penaeid Shrimps in reproduction phase. Revista Eletrônica Científica Centauro 1(2):58–65
Qin M, Mou X-J, Dong W-H, Liu J-X, Liu H, Dai Z, Huang X-W, Wang N, Yan X (2020) In situ electrospinning wound healing films composed of zein and clove essential oil. Macromol Mater Eng. https://doi.org/10.1002/mame.201900790
He H, Kara Y, Molnar K (2019) In situ viscosity-controlled electrospinning with a low threshold voltage. Macromol Mater Eng. https://doi.org/10.1002/mame.201900349
Wang Y, Chen L (2012) Electrospinning of prolamin proteins in acetic acid: the effects of protein conformation and aggregation in solution. Macromol Mater Eng 297:902–913. https://doi.org/10.1002/mame.201100410
Aygun B, Ozdemir H, Oksuzomer MAF (2019) Structural, morphological and conductivity properties of samaria doped ceria (SmxCe1-xO2-x/2) electrolytes synthesized by electrospinning method. Mater Chem Phys 232:82–87. https://doi.org/10.1016/j.matchemphys.2019.04.067
Naragund VS, Panda PK (2020) Electrospinning of cellulose acetate nanofiber membrane using methyl ethyl ketone and N, N-dimethylacetamide as solvents. Mater Chem Phys. https://doi.org/10.1016/j.matchemphys.2019.122147
Abedi A, Hasanzadeh M, Tayebi L (2019) Conductive nanofibrous Chitosan/PEDOT: PSS tissue engineering scaffolds. Mater Chem Phys. https://doi.org/10.1016/j.matchemphys.2019.121882
Torres-Giner S, Gimenez E, Lagarona JM (2008) Characterization of the morphology and thermal properties of zein prolamine nanostructures obtained by electrospinning. Food Hydrocoll 22:601–614. https://doi.org/10.1016/j.foodhyd.2007.02.005
Pereda M, Arnica G, Racz I, Marcovich NE (2011) Structure and properties of nanocomposite films based on sodium caseinate and nanocellulose fibers. J Food Eng 103:76–83. https://doi.org/10.1016/j.jfoodeng.2010.10.001
Karuppannan C, Sivaraj M, Kumar JG, Seerangan R, Balasubramanian S, Gopal DR (2017) Fabrication of progesterone-loaded nanofibers for the drug delivery applications in bovine. Nanoscale Res Lett. https://doi.org/10.1186/s11671-016-1781-2
Lu H, Wang Q, Li G, Qiu Y, Wei Q (2017) Electrospun water-stable zein/ethyl cellulose composite nanofiber and its drug release properties. Mater Sci Eng C-Mater Biol Appl 74:86–93. https://doi.org/10.1016/j.msec.2017.02.004
Yang S, Dai L, Mao L, Liu J, Yuan F, Li Z, Gao Y (2019) Effect of sodium tripolyphosphate incorporation on physical, structural, morphological and stability characteristics of zein and gliadin nanoparticles. Int J Biol Macromol 136:653–660. https://doi.org/10.1016/j.ijbiomac.2019.06.052
Ansari AQ, Ansari SJ, Khan MQ, Khan MF, Qureshi UA, Khatri Z, Ahmed F, Kim IS (2019) Electrospun zein nanofibers as drug carriers for controlled delivery of Levodopa in Parkinson syndrome. Mater Res Express 6:075405. https://doi.org/10.1088/2053-1591/ab16bf
Ayodhya D, Venkatesham M, Santoshi KA, Bhagavanth RG, Veerabhadram G (2015) One-pot sonochemical synthesis of CdS nanoparticles: photocatalytic and electrical properties. Int J Ind Chem 6:261–271. https://doi.org/10.1007/s40090-015-0047-7
Oliveira JE, Mattoso LHC, Orts WJ, Medeiros ES (2013) Structural and morphological characterization of micro and nanofibers produced by electrospinning and solution blow spinning: a comparative study. Adv Mater Sci Eng. https://doi.org/10.1155/2013/409572
Nam K-M, Mees K, Park H-S, Willert-Porada M, Lee C-S (2014) Electrophoretic deposition for the growth of carbon nanofibers on Ni-Cu/C-fiber textiles. Bull Korean Chem Soc 35:2431–2437. https://doi.org/10.5012/bkcs.2014.35.8.2431
Kayaci F, Uyar T (2012) Electrospun zein nanofibers incorporating cyclodextrins. Carbohydr Polym 90:558–568. https://doi.org/10.1016/j.carbpol.2012.05.078
Ullah S, Hashmi M, Khan MQ, Kharaghani D, Saito Y, Yamamoto T, Kim IS (2019) Silver sulfadiazine loaded zein nanofiber mats as a novel wound dressing. RSC Adv 9:268–277. https://doi.org/10.1039/c8ra09082c
You F, Wang D, Li X, Liu M, Hu G-H, Dang Z-M (2014) Interfacial engineering of polypropylene/graphene nanocomposites: improvement of graphene dispersion by using tryptophan as a stabilizer. RSC Adv 4:8799–8807. https://doi.org/10.1039/c3ra47112h
Scramin JA, de Britto D, Forato LA, Bernardes-Filho R, Colnago LA, Assis OBG (2011) Characterisation of zein-oleic acid films and applications in fruit coating. Int J Food Sci Technol 46:2145–2152. https://doi.org/10.1111/j.1365-2621.2011.02729.x
Falco I, Flores-Meraz PL, Randazzo W, Sanchez G, Lopez-Rubio A, Jose FM (2019) Antiviral activity of alginate-oleic acid based coatings incorporating green tea extract on strawberries and raspberries. Food Hydrocoll 87:611–618. https://doi.org/10.1016/j.foodhyd.2018.08.055
Brahatheeswaran D, Mathew A, Aswathy RG, Nagaoka Y, Venugopal K, Yoshida Y, Maekawa T, Sakthikumar D (2012) Hybrid fluorescent curcumin loaded zein electrospun nanofibrous scaffold for biomedical applications. Biomed Mater 7:045001. https://doi.org/10.1088/1748-6041/7/4/045001
Corradini E, Mattoso L, Guedes C, Rosa D (2004) Mechanical, thermal and morphological properties of poly (epsilon-caprolactone)/zein blends. Polym Adv Technol 15:340–345. https://doi.org/10.1002/pat.478
Hosseini SF, Zandi M, Rezaei M, Farahmandghavi F (2013) Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: preparation, characterization and in vitro release study. Carbohydr Polym 95:50–56. https://doi.org/10.1016/j.carbpol.2013.02.031
Pandey H, Parashar V, Parashar R, Prakash R, Ramteke PW, Pandey AC (2011) Controlled drug release characteristics and enhanced antibacterial effect of graphene nanosheets containing gentamicin sulfate. Nanoscale 3:4104–4108. https://doi.org/10.1039/C1NR10661A
Singhvi G, Singh M (2011) Review: in vitro drug release characterization models. Int J Pharm Stud Res 2:77–84
Hayat U, Raza A, Wang H-J, Wang J-Y (2020) Preparation of ciprofloxacin loaded zein conduits with good mechanical properties and antibacterial activity. Mater Sci Eng, C 111:110766. https://doi.org/10.1016/j.msec.2020.110766
Raza A, Hayat U, Wang H-J, Wang J-Y (2020) Preparation and evaluation of captopril loaded gastro-retentive zein based porous floating tablets. Int J Pharm 579:119185. https://doi.org/10.1016/j.ijpharm.2020.119185
Liu W, Kong Y, Ye A, Shen P, Dong L, Xu X, Hou Y, Wang Y, Jin Y, Han J (2020) Preparation, formation mechanism and in vitro dynamic digestion behavior of quercetin-loaded liposomes in hydrogels. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2020.105743
Duan X-D, Feng L, Jiang W-D, Wu P, Liu Y, Jiang J, Tan B-P, Yang Q-H, Kuang S-Y, Tang L, Zhou X-Q (2020) The dynamic process of dietary soybean β-conglycinin in digestion, absorption, and metabolism among different intestinal segments in grass carp (Ctenopharyngodon idella). Fish Physiol Biochem. https://doi.org/10.1007/s10695-020-00794-9
Kleemann C, Schuster R, Rosenecker E, Selmer I, Smirnova I, Kulozik U (2020) In-vitro-digestion and swelling kinetics of whey protein, egg white protein and sodium caseinate aerogels. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2019.105534
Du Le H, Loveday SM, Singh H, Sarkar A (2020) Gastrointestinal digestion of pickering emulsions stabilised by hydrophobically modified cellulose nanocrystals: release of short-chain fatty acids. Food Chem. https://doi.org/10.1016/j.foodchem.2020.126650
Hu M-X, Li J-N, Guo Q, Zhu Y-Q, Niu H-M (2019) Probiotics biofilm-integrated electrospun nanofiber membranes: a new starter culture for fermented milk production. J Agric Food Chem 67:3198–3208. https://doi.org/10.1021/acs.jafc.8b05024
Chen J, Xiao J, Wang Z, Cheng H, Zhang Y, Lin B, Qin L, Bai Y (2020) Effects of reaction condition on glycosidic linkage structure, physical-chemical properties and in vitro digestibility of pyrodextrins prepared from native waxy maize starch. Food Chem. https://doi.org/10.1016/j.foodchem.2020.126491
Acknowledgements
The authors are grateful for the financial support from FINEP/MCT, CAPES, CNPq (403357/2016-0, 302469/2018-4), FAPEMIG (APQ-01505-15, APQ-00906-17), Embrapa/LNNA/Rede AgroNano.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva, S.C.M., Fuzatto, R.H.S., Botrel, D.A. et al. Development of zein nanofibers for the controlled delivery of essential amino acids for fish nutrition. SN Appl. Sci. 2, 1783 (2020). https://doi.org/10.1007/s42452-020-03616-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03616-y