Abstract
A hydrogeochemical assessment was carried out in Jirania Block of west district of Tripura, India, to evaluate the geochemical processes and groundwater suitability for drinking and irrigation purposes. Twenty-two groundwater samples were collected at different lithologies in the study area during post-monsoon period. The collected samples were analyzed for major ions and heavy metals using the standard protocols. The analyzed parameters were used for various geochemical plots and indices to classify the groundwater quality. The order of dominance of cations is Ca2+ > Mg2+ > Na+ > K+ and HCO3− > Cl− > SO42− > NO3− for anions. Higher concentration of Fe and Mn was observed in most of the samples, which are above the acceptable limit of BIS standard. Correlation analysis and factor analysis were applied to unravel the relationship between ions and to identify the possible geochemical processes in groundwater. The interpretation of analytical data, plots and indices reveals that the weathering, mineral dissolution and ion exchange reactions are the major controlling factors for the groundwater geochemistry. The quality of the groundwater is suitable for drinking and irrigation purposes to the local community in this assessment period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Groundwater is an important source of water supply for domestic, industrial, agricultural and recreational activities in both urban and rural places, and one-third of the world’s population is using groundwater for drinking purpose [1,2,3,4]. Less bacteriological contamination and occurrence of diverse health necessity nutrients makes the groundwater more reliable in contrast to surface water. The suitability of groundwater is depending upon its steady temperature, natural quality, availability and low susceptibility. The quality of groundwater is also related with various geological and chemical influences that include precipitation, recharge quality, water–rock relation, dissolution, mineralization, ion exchange, etc [3, 5].
Groundwater is one of the prime water supply sources for drinking and agricultural purposes found in most parts of India. A survey of World Bank together with Central Groundwater Board has reflected that India is the world’s largest user of groundwater and major fresh groundwater resources of the country is to be utilizable [6,7,8]. Groundwater pollution is a serious issue all over the world in recent period. Intensive use of groundwater with respect to urbanization, industrialization and agricultural activity has affected both quality and quantity of groundwater imposing greater risk to the groundwater resources. When pollution comes into the subsurface environment, it is distributed over the broad area of groundwater aquifer and makes it unsuitable for consumption and other various utilizations.
In India, it has been found that about 21% of transmissible diseases are water related [9,10,11]. Cardiovascular diseases, problems in nervous system and multiple organ troubles are greatly connected to the consumption of polluted drinking water. It has been found that higher intake of calcium, magnesium, potassium and sodium give serious impact on hypertension and cardiovascular diseases in humans [12]. Higher concentration of fluoride through drinking water creates disorders in skeletal structure, bladder and uterine cancer. Elevated concentration of different types of toxic heavy metals in food and water is unsafe to human health [13].
In adults, arsenic is the most worried issue that cause of acute heavy metal poisoning. Human exposure to arsenic can cause cancer, hyperkeratosis, cardiovascular and nervous system problems. Consumption of chromium through food and water is also very unsafe and harmful, critically damaging lungs and kidneys. High concentrations of lead in drinking water make carcinogenic effects for humans. Ingestion caused by iron in drinking water is another metal pollution alarm especially for young kids as iron rapidly absorbed in gastrointestinal tract. High level of iron exposure can also cause bad effects to liver and kidneys [14].
Asghari et al. [15] have used the physical and chemical elements of groundwater in Zanjan area, Iran, to identify the suitability for drinking purpose, and they found that the groundwater is within the WHO standards and appropriate for drinking. Similar study was carried out by Soleimani et al. [16] in Sarpol-e Zahab area, Iran, and found that higher water hardness was observed in most of the samples.
The hydrochemistry of groundwater provides important facts in order to observe the suitability of water for both domestic and drinking purposes. The facts include physiochemical variation in groundwater of a particular area that is greatly affected by the local geology, geological structures and anthropogenic impacts [6]. Significant studies on hydrogeochemistry of groundwater have been investigated in various parts of India [2, 17,18,19,20,21,22,23,24,25].
The main aim of the current research is to evaluate the hydrochemistry of groundwater for drinking and irrigation purposes in Jirania Block of West Tripura District, Tripura, India, where groundwater is a prime source for the local people. Through the literature survey, it is found that there is no systematic work on hydrogeochemistry of groundwater in this area. Thus, it is important to estimate the extent to which the water consumed by the residents of rural areas has been polluted. The important significance of the study is that the majority of the population of this area belongs to tribal community where people are less aware about the health issues caused by poor quality of water due to poor literacy rate and general awareness. Based on the above facts, the groundwater quality of this region is prime important. Hence, the objective of this research is to use the hydrogeochemical tool to assess the suitability of groundwater in terms of domestic and irrigation purposes. This study also aims at (includes) the understanding of the various geochemical processes which alter the groundwater quality in the study area.
2 Study area
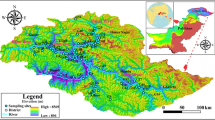
This study was conducted at Jirania Block under the West Tripura District in the State Tripura, India. The area is geographically bound by latitude between 23°40′0″N and 23°55′0″N and longitude between 91°20′0″E and 91°30′0″E with an average altitude of 45 m above the MSL (Fig. 1). It is located about 18 km away from Agartala, the state capital of Tripura, and it is connected by the national highway NH-44. The climate of study area is tropical monsoon type. The average annual rainfall is around 218 cm, and it receives maximum rainfall from South-west monsoon, which remains in the month of June to September. The total geographic area is 212.32 km2, and the total population of Jirania is 55,873. According to the land use/cover in the study area, natural vegetation covers 101.93 km2 followed by agriculture (86.38 km2), settlement (16.48 km2), water body (5.18 km2) and barren land (2.35 km2).
2.1 Geology and lithology
The study area consists of three main geological Formations viz, Tipam, Dupitila and Bokabil and also the Alluvium deposit along the banks of Haora River in the west district (Fig. 1). Tipam Formation mainly consists of sandstones, which is soft and fragile and associated with alternating layers of clay/mud [26]. Dupitila Formation overlaid by Tipam Formation, and it consists of shaley sandstone. Bokabil Formation overlaid by Dupitila Formation and mainly consists of shale with sandstone and limestone beds. This succession consists of some primary sedimentary structures. Quartz, feldspars and micas are the major mineral content in those rocks.
In Tripura, all the formations are comprised of sedimentary rocks with good porosity. These rocks are considered as potential aquifers and conduits for groundwater movement, in which the groundwater can be pumped for various purposes [26]. Groundwater in hilly regions of Tripura is the noticeable solution for public water supply. The major lithological units in the study area range from sandstone in Tipam Formation to shale–sandstone in Bokabil Formation as shown in Fig. 1. The sandstones are medium to fine grained. Alluvium deposit along the banks of seasonal rivers is represented by unconsolidated silt, sand, clay and decomposed organic matters.
3 Methodology
Twenty-two groundwater samples were collected in Jirania Block (Fig. 1) during post-monsoon period. One liter capacity of polyethylene (HDPE) bottles was used to collect the samples. Before sampling, bottles were washed thoroughly with dilute HNO3 acid and then with distilled water. Quality control measures were adopted throughout the sampling to meet the Quality Assurance (QA) [27]. pH, electrical conductivity (EC) and total dissolved solids (TDS) were measured in the field using multi-probe water quality meter.
Ca2+, Mg2+, HCO3− and Cl−were analyzed using titrimetric method [27]. Na+ and K+ were measured by atomic absorption spectrophotometer (PerkinElmer AA-Analyst 700). SO42− was measured by turbidimetric method using BaSO4, and NO3− was measured using UV visible spectrophotometer.
To confirm the analytical accuracy for samples, ionic balance error was computed using cation and anion concentrations [28]. The error percentage for the samples is within the standard limit of ± 5%.
For heavy metals analysis, samples were acidified using HNO3 by bringing the pH to ~ 2 and preserved at a temperature of 4 °C. Fe, Mn, Pb, Cd, As, Cu and Zn were analyzed using atomic absorption spectrophotometer (PerkinElmer A Analyst 700). Most of the samples show below detection limit for Pb, Cd and As. Hence, these metals were not considered for further discussion. The minimum detection limit for Fe, Mn, Cu and Zn is 0.001 mg/L.
WATCLAST Program [29] was used for the analytical data to classify the groundwater based on geochemical indices and for graphical representation. Log pCO2, ionic strength and the saturation index of carbonate minerals were calculated using WATQ4F geochemical modeling software [30]. Correlation and factor analysis were used for interpreting the hydrochemical data set using Statistical Package for Social Studies (SPSS) version 17. Maps and spatial diagrams were created using ArcGIS version 10.1.
4 Results and discussion
4.1 General chemistry
Table 1 shows the physical and chemical composition of groundwater in the study area. pH in water is an important indicator for quality and also for geochemical reactions [31]. pH in the groundwater samples ranging from 5.68 to 7.83 with an average of 6.53. It indicates that the groundwater is near neutral condition and few samples in acidic nature. This kind of water may aggravate the dissolution of minerals during weathering process. EC values range from 75.1 to 260 µS/cm with an average of 160.94 µS/cm. According to Wilcox [32] classification, all the samples fall in excellent category, except one sample. The spatial distribution of EC values shows that the higher values are noted in the western side of the study area (Fig. 2). TDS values range from 48 to 152 mg/L with an average of 93.6 mg/L, and it follows the same spatial trend of EC. Ca2+ is the dominant cation ranging from 5.9 to 22.47 mg/L with an average of 11.93 mg/L, followed by Mg2+, Na+ and K+. HCO3− is the dominant anion ranging from 40.32 to 176.4 mg/L with an average of 97.6 mg/L, followed by Cl−, SO42− and NO3−.
4.2 Hydrogeochemical processes
Piper plot [33] was used to categorize the major water types in the study area. In this plot, most of the samples fall in Ca–HCO3 water type (Fig. 3), indicating the dominance of weathering process by the fresh water recharge [2]. It also shows that the water–rock interaction is the major process in the study area. Some minor representations are in Ca–Cl water type. Overall, the plot shows that the alkaline earth (Ca2+ and Mg2+) exceeds alkali (Na+) and weak acids (HCO3−) exceeds the strong acid (Cl−).
Gibbs plot [34] was also used to identify the major hydrogeochemical processes viz weathering, evaporation and precipitation, which may control the groundwater chemistry. This plot consists of two separate diagrams for cations and anions. Majority of the samples fall in weathering zone, indicating the water–rock interaction dominance [35]. Few samples fall in precipitation zone (Fig. 4).
The concentration of ions and their interrelationships is mainly used for the chemical classification. The plot (Ca + Mg) versus HCO3 shows that most of the samples fall below equiline 1:1 indicating the predominance of bicarbonate over alkali earth (Fig. 5). This is due to the intensive weathering of rocks. The (Ca + Mg) versus TZ+ plot lies below the equiline 1:1 indicating the dominant of total cations over alkali earth. The plot for (Na + K) versus TZ+ also indicates the dominance of total cations over alkali earth. Na versus Cl plot shows that the majority of samples fall in Cl zone indicating the leaching of secondary salts [36].
Since weathering is the chief controlling process in the groundwater, Chloro-alkaline indices, i.e., CAI1 and CAI2 were used to assess the degree of base exchange during weathering [37]. During water–rock interaction, if there is an exchange of alkali elements (Na+ and K+) in water with alkaline earth (Ca2+ or Mg2+) in rock, then both indices are positive and vise versa. In this calculation, all the ionic concentration is expressed in epm values. Based on the calculation, positive values are observed in most of the samples indicating reverse ion exchange process during weathering (Table 2). In Schoeller [38], classification of water types, the majority of samples fall in type I and few samples are of type II, indicating that the chemistry of groundwater is ruled by residence time of water in the rock matrix and extent of water–rock interaction [21].
Log pCO2 and ionic strength are used to assess the nature of groundwater in the sense of residence time in the aquifer [39]. In fresh water, the ionic strength is less than 0.005 [31] and all the samples in the study area show lower ionic strength indicating higher inflow of fresh water recharge (Fig. 6). In Log pCO2, all the samples are higher than the atmospheric value (− 3.5) suggesting longer residence time in the aquifer, which enhance the water–rock interaction [40].
Geochemical model, WATEQ4F [30], was used to calculate the saturation index (SI) of carbonate minerals in the groundwater. The results show that high SI of calcite followed by magnesite, anhydrite and dolomite (Fig. 7). All the samples fall under saturation state indicating the dissolution of minerals during water–rock interaction [41].
4.3 Water quality
The analyzed parameters are compared with BIS [42] and WHO [43] standards to determine the drinking water quality (Table 3). All the ions are within the permissible limits of WHO standard except pH, in which 13 samples are less than 6.5. HCO3 values for all the samples are shown above the acceptable range of BIS standard.
Based on chloride classification by Stuyfzand [44], the majority of samples fall in very fresh and few samples in fresh category (Table 2). For corrosivity ratio, most of the samples fall in safer zone, which is less than 1. Carbonate hardness (CH) is higher in most of the samples indicating temporary hardness. According to Sawyer and McCarty [45] hardness, samples vary from soft to slightly hard (Table 2).
Groundwater suitability for irrigation purpose is estimated by Na%, EC, sodium absorption ratio (SAR) and residual sodium carbonate (RSC) [32, 46]. Na concentration is an important indicator to determine the soil structure and crop yield [47]. In Na%, samples range from excellent to good category and in Na% Eaton [48] classification; all the samples are in safe category (Table 2). SAR values in all the samples fall in excellent category (Table 2). In residual sodium carbonate [46] classification, all samples are in good category except one sample in medium category, indicating that the water is fit for irrigation.
USSL plot is used to classify the irrigation water quality (Fig. 8). In the plot, most of the samples fall in C1S1 zone indicating low salinity and low sodium hazard, which is suitable for plants growth. Permeability index is used to determine the quality of irrigation water based on soil texture for development in agriculture. Based on Doneen [49] plot using permeability index values, groundwater is classified into class I, class II and class III to determine the suitability of water for irrigation purpose (Fig. 9). In this plot, samples are equally distributed in class I and II, which indicate good permeability and the water can be used for irrigation.
Table 4 shows the comparison of present study chemical composition of groundwater with few other parts of world and India regions [50,51,52,53,54,55,56,57,58,59,60]. It is clearly noticed that the chemical concentration of West Tripura (present study) is much lower than the world and India regions. It is also interesting to observe that the chemical concentration of groundwater of Nalbari district of Assam (which is close to the state of Tripura) is lower than the other parts of world and India regions. But, most of the chemical elements of present study are lower than the groundwater of Nalbari district of Assam, except Cl and SO4. The higher concentration of Cl and SO4 in the study area indicates the leaching of secondary salts and weathering of host rocks [17].
4.4 Heavy metals
The heavy metal concentration shows significant variation in each sample (Fig. 10). Fe ranges from 120 to 5140 ppb with an average of 2692 ppb and 90% of samples fall above the BIS maximum acceptable limit (300 ppb). Mn ranges from 11 to 619 ppb with an average of 125 ppb. 40% of the samples falls above the BIS maximum acceptable limit (100 ppb). Cu ranges from 10 to 38 ppb with an average of 15 ppb, whereas Zn ranges from 13 to 50 ppb with an average of 27 ppb. All the samples fall well below the BIS maximum acceptable limit of Cu (50 ppb) and Zn (5000 ppb) [42]. Higher concentration of Fe and Mn is due to the weathering of bedrocks, which release metals into the groundwater [61].
4.5 Statistical analysis
4.5.1 Correlation analysis
Correlation analysis was used for the groundwater samples to reveal the inter-relationship between ions [22, 24, 36]. Correlation coefficient > 0.5 considered as good correlation between ions. pH and SO42− show good correlation indicating the dissolution of sulfate minerals during weathering process (Table 5). HCO3− had a good correlation between EC, TDS, Na+ and K+ indicating the dominance of HCO3− ion in groundwater due to weathering and dissolution of minerals [2]. Ca2+ and Mg2+ showed good correlation indicating the dissolution of carbonate minerals from the host rocks or simple cation exchange reaction.
4.5.2 Factor analysis
Factor analysis was also used for the groundwater samples to determine the various geochemical process indicated by the factor loading of ions [20, 21]. Totally, four factors were extracted with the total variance of 24%, 22%, 19% and 17% (Table 6). Factor 1 is loaded with EC, TDS and HCO3−, indicating that the overall chemistry of groundwater is mainly controlled by HCO3− ion and also showing the indicative of dominant mineral dissolution to increase the HCO3 concentration. Factor 2 is loaded with Na+ and K+ indicating the feldspar mineral weathering from the source rock [62]. Factor 3 is loaded with Ca2+ and Mg2+ indicating the reverse ion exchange reaction, where the Ca and Mg are released from mineral weathering [58]. Factor 4 is loaded with pH and SO42− indicating the dissolution of sulfate minerals during weathering.
5 Conclusions
The outcome of this study has given a better insight on the nature of groundwater in terms of quality and the geochemical processes in the study area. Most of the samples show slightly acidic in nature, which enhance the dissolution of secondary minerals during water–rock interaction. Ca–HCO3 is the major water type in Piper plot indicating the dominance of weathering process through fresh water recharge. Gibbs plot also shows the weathering is the major controlling process in groundwater. Higher Log pCO2 values support the longer residence time of water in the aquifer matrix, which enhance the dissolution and ion exchange reactions. Saturation index of carbonates minerals shows the under-saturation condition, which again supports the mineral dissolution during weathering. The heavy metals in the groundwater are mostly contributed from the weathering of rock forming minerals. Factor analysis indicates weathering, dissolution and ion exchange are the dominant processes that control the hydrogeochemistry of groundwater. Overall, the groundwater quality is suitable for drinking and irrigation purposes, except for the cautious of high level Fe, Mn and HCO3.
References
Das M, Kumar A, Mohapatra M, Murali SD (2010) Evolution of drinking quality of groundwater through multivariate techniques in urban area. Environ Monit Assess 166(1–4):149–157
Thilagavathai R, Chidambaram S, Thivya C, Prasanna MV, Singaraja C, Tirumalesh K, Pethaperumal S (2014) Delineation of natural and anthropogenic process controlling hydrogeochemistry of layered aquifer sequence. Proc Natl Acad India A Phys Sci 84(1):95–108
Nag SK, Das S (2017) Assessment of groundwater quality from Bankura I and II Blocks, Bankura District, West Bengal, India. Appl Water Sci. https://doi.org/10.1007/s13201-017-0530-8
Selvakumara S, Chandrasekara N, Kumar G (2017) Hydrogeochemical characteristics and groundwater contamination in the rapid urban development areas of Coimbatore, India. Water Resour Ind 17:26–33
Wagh VM, Panaskar DB, Muley AA, Mukate SV (2017) Groundwater suitability evaluation by CCME WQI model for Kadava River Basin, Nashik, Maharashtra, India. Model Earth Syst Environ. https://doi.org/10.1007/s40808-017-0316-x
Prasanth SV, Magesh NS, Jitheshlal KV, Chandrasekar N, Gangadhar K (2012) Evaluation of groundwater quality and its suitability for drinking and agricultural use in the coastal stretch of Alappuzha District, Kerala, India. Appl Water Sci. https://doi.org/10.1007/s13201-012-0042-5
Singh N, Singh RP, Mukherjee S, McDonald K, Reddy KJ (2014) Hydrogeological processes controlling the release of arsenic in parts of 24 Parganas district, West Bengal. Environ Earth Sci 72(1):111–118
Kaviarasan M, Geetha P, Soman KP (2016) GIS-based ground water quality monitoring in Thiruvannamalai District, Tamil Nadu, India. In: Proceedings of the international conference on soft computing systems, advances in intelligent systems and computing, vol 397, pp 685–700
Jerome C, Pius A (2010) Evaluation of Water Quality Index and its impact on the quality of life in an industrial area in Bangalore, South India. Am J Sci Ind Res 1(3):595–603
Sharma DA, Rishi MS, Keesari T (2016) Evaluation of groundwater quality and suitability for irrigation and drinking purposes in southwest Punjab, India using hydrochemical approach. Appl Water Sci. https://doi.org/10.1007/s13201-016-0456-6
Shanmugasundharam A, Kalpana G, Mahapatra SR, Sudharson ER, Jayaprakash M (2017) Assessment of Groundwater quality in Krishnagiri and Vellore Districts in Tamil Nadu, India. Appl Water Sci 7:1869–1879
Yousefi M, Najafi Saleh H, Yaseri M, Jalilzadeh M, Mohammadi AA (2018) Association of consumption of excess hard water, body mass index and waist circumference with risk of hypertension in individuals living in hard and soft water areas. Environ Geochem Health. https://doi.org/10.1007/s10653-018-0206-9
Moghaddam VK, Yousefi M, Khosravi A, Yaseri M, Mahvi AH, Hadei M, Mohammadi AA, Robati Z, Mokammel A (2018) High concentration of fluoride can be increased rick of abortion. Biol Trace Elem Res 185:262–265
Saleh HN, Panahande M, Yousefi M, Asghari FB, Conti GO, Talaee E, Mohammadi AA (2018) Carcinogenic and non-carcinogenic risk assessment of heavy metals in groundwater wells in Neyshabur Plain, Iran. Biol Trace Elem Res. https://doi.org/10.1007/s12011-018-1516-6
Asghari FB, Mohammadi AA, Dehghani MH, Yousefi M (2018) Data on assessment of groundwater quality with application of ArcGIS in Zanjan, Iran. Data Brief 18:375–379
Soleimani H, Abbasnia A, Yousefi M, Mohammadi AA, Khorasgani FC (2018) Data on assessment of groundwater quality for drinking and irrigation in rural area Sarpol-e Zahab city, Kermanshah province, Iran. Data Brief 17:148–156
Prasanna MV, Chidambaram S, Shahul Hameed A, Srinivasamoorthy K (2010) Study of evaluation of groundwater in Gadilam basin using hydrogeochemical and isotope data. Environ Monit Assess 168:63–90
Krishna Kumar S, Chandrasekar N, Seralathan P, Godson PS, Magesh NS (2011) Hydrogeochemical study of shallow carbonate aquifers, Rameswaram Island, India. Environ Monit Assess. https://doi.org/10.1007/s10661-011-2249-6
Chidambaram S, Prasanna MV, Karmegam U, Singaraja C, Pethaperumal S, Manivannan R, Anandhan P, Tirumalesh K (2011) Significance of pCO2 values in determining carbonate chemistry in groundwater of Pondicherry region, India. Front Earth Sci 5(2):197–206
Vasanthavigar M, Srinivasamoorthy K, Prasanna MV (2013) Identification of groundwater contamination zones and its sources by using multivariate statistical approach in Thirumanimuthar sub-basin, Tamil Nadu, India. Environ Earth Sci 68:1783–1795
Singaraja C, Chidambaram S, Prasanna MV, Thivya C, Thilagavathi R (2014) Statistical analysis of the hydrogeochemical evolution of groundwater in hard rock aquifers of Thoothukudi district in Tamil Nadu, India. Environ Earth Sci 71:451–464
Thivya C, Chidambaram S, Thilagavathi R, Prasanna MV, Singaraja C, Adithya VS, Nepolian M (2015) A multivariate statistical approach to identify the spatio-temporal variation of geochemical process in a hard rock aquifer. Environ Monit Assess 187:552
Adithya VS, Chidambaram S, Thivya C, Thilagavathi R, Prasanna MV, Nepolian M, Ganesh N (2016) A study on the impact of weathering in groundwater chemistry of a hard rock aquifer. Arab J Geosci 9:158
Thilagavathi R, Chidambaram S, Thivya C, Prasanna MV, Tirumalesh K, Pethaperumal S (2017) Assessment of groundwater chemistry in layered coastal aquifers using multivariate statistical analysis. Sustain Water Resour Manag 3:55–69
Panda Banaja Rani, Chidambaram S, Ganesh N, Adithya VS, Prasanna MV, Pradeep K, Vasudevan U (2018) A hydrochemical approach to estimate mountain front recharge in an aquifer system in Tamilnadu, India. Acta Geochem 37:465–488
GSI (2011) Geology and mineral resources of Manipur, Mizoram, Nagaland and Tripura. Geological Survey of India. Miscellaneous Publication, No. 30 Part IV Vol:1 (Part-2)
APHA (2012) Standard methods for the examination of water and wastewater, 22nd edn. APHA, Washington
Domenico PA, Schwartz FW (1990) Physical and chemical hydrogeology. Wiley, New York, pp 410–420
Chidambaram S, Ramanathan AL, Srinivasamoorthy K, Anandhan P (2003) WATCLAST—a computer program for hydrogeochemical studies. In: Recent trends in hydrogeochemistry (case studies from surface and subsurface waters of selected countries). Capital Publishing Company, New Delhi, pp 203–207
Ball JW, Nordstrom DK (1992) User’s manual for WATEQ4F, with revised thermodynamic database and test cases for calculating speciation of minor, trace and redox elements in natural waters. US Geological Survey, Open File Report 91-183
Hem JD (1959) Geochemistry of water calculation and use of ion activity. USGS water supply, 1935 C
Wilcox LV (1955) Classification and use of irrigation water. US Geological Department Agriculture Circular, 969
Piper AM (1944) A graphic procedure in geochemical interpretation of water analysis. Trans Geophys Union 25:914–923
Gibbs RJ (1970) Mechanism controlling world water chemistry. Science 170:1088–1090
Nagarajan R, Rajmohan N, Mahendran U, Senthamilkumar S (2010) Evaluation of groundwater quality and its suitability for drinking and agricultural use in Thanjavur city, Tamil Nadu, India. Environ Monit Assess 171:289–308
Prasanna MV, Chidambaram S, Srinivasamoorthy K (2010) Statistical analysis of the hydrogeochemical evolution of groundwater in hard and sedimentary aquifers system of Gadilam river basin, South India. J King Saud Univ Sci 22:133–145
Schoeller H (1965) Hydrodynamic dams lekar collogue. Doboronik 1:3–20
Schoeller H (1967) Qualitative evaluation of groundwater resources. In: Methods and techniques of ground water investigation and development. Water Res Series-33 UNESCO, pp 44–52
Chidambaram S, Karmegam U, Prasanna MV, Sasidhar P, Vasanthavigar M (2011) A study on hydrochemical elucidation of coastal groundwater in and around Kalpakkam region, Southern India. Environ Earth Sci 64:1419–1434
Prasanna MV, Chidambaram S, Gireesh TV, Jabir Ali TV (2011) A study on hydrochemical characteristics of surface and sub-surface water in and around Perumal Lake, Cuddalore district, Tamil Nadu, South India. Environ Earth Sci 63:31–47
Srinivasamoorthy K, Chidambaram S, Prasanna MV, Vasanthavihar M, John P, Anandhan P (2008) Identification of major sources controlling groundwater chemistry from a hard rock terrain—a case study from Mettur taluk, Salem district, Tamil Nadu, India. J Earth Syst Sci 117:49–58
BIS (2012) Drinking water specification, Second Revision, Bureau of Indian Standards, Manak Bhawan, 9, Bahadur Shah Zafar Marg, New Delhi
WHO (2017) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva, p 541
Stuyfzand PJ (1989) Nonpoint sources of trace elements in potable groundwaters in the Netherlands. In: Proceedings 18th TWSA Water Workings. Testing and Research Institute KIWA
Sawyer CN, McCarty PL (1967) Chemistry for sanitary engineers. McGraw-Hill, New York
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. USDA Handbook, 60
Srinivasamoorthy K (2005) Hydrogeochemistry of groundwater in Salem district of Tamilnadu, India. Unpublished Ph.D. Thesis, Annamalai University, India
Eaton EM (1950) Significance of carbonate in irrigation water. Soil Sci 69:123–133
Doneen LD (1948) The quality of irrigation water. Calif Agric Dep 4(11):6–14
Venkatramanan S, Chung SY, Rajesh R, Lee SY, Ramkumar T, Prasanna MV (2015) Comprehensive studies of hydrogeochemical processes and quality status of groundwater with tools of cluster, grouping analysis, and fuzzy set method using GIS platform: a case study of Dalcheon in Ulsan City, Korea. Environ Sci Pollut Res 22:11209–11223
Ayed B, Jmal I, Sahal S, Mokadem N, Saidi S, Boughariou E, Bouri S (2017) Hydrochemical characterization of groundwater using multivariate statistical analysis: the Maritime Djeffara shallow aquifer (Southeastern Tunisia). Environ Earth Sci 76:821
Aghazadeh N, Chitsazan M, Golestan Y (2017) Hydrochemistry and quality assessment of groundwater in the Ardabil area, Iran. Appl Water Sci 7:3599–3616
Emenike PC, Tenebe IT, Nnaji CC (2018) Assessment of geospatial and hydrochemical interactions of groundwater quality, southwestern Nigeria. Environ Monit Assess 190:440
Elubid BA, Tao Huang, Ahmed EH, Zhao J, Elhag KM, Abbas W, Babiker MM (2019) Geospatial distributions of groundwater quality in Gedaref State using geographic information system (GIS) and drinking water quality index (DWQI). Int J Environ Res Public Health 16:731
Bhuiyan MAH, Bodrud-Doza M, Towfiqul Islam ARM, Rakib MA, Safiur Rahman M, Ramanathan AL (2016) Assessment of groundwater quality of Lakshimpur district of Bangladesh using water quality indices, geostatistical methods and multivariate analysis. Environ Earth Sci 75:1020
Roy A, Keesari T, Mohokar H, Sinha UK, Bitra S (2018) Assessment of groundwater quality in hard rock aquifer of central Telangana state for drinking and agricultural purposes. Appl Water Sci 8:124
Kaur T, Bhardwaj R, Arora S (2017) Assessment of groundwater quality for drinking and irrigation purposes using hydrochemical studies in Malwa region, southwestern part of Punjab, India. Appl Water Sci 7:3301–3316
Adithya VS, Chidambaram S, Thivya C, Thilagavathi R, Prasanna MV, Nepolian M, Ganesh N (2016) A study on the impact of weathering in groundwater chemistry of a hard rock aquifer. Arabian J Geosci 9:158
Subba Rao N (2018) Groundwater quality from a part of Prakasam District, Andhra Pradesh, India. Appl Water Sci 8:30
Jain CK, Vaid U (2018) Assessment of groundwater quality for drinking and irrigation purposes using hydrochemical studies in Nalbari district of Assam, India. Environ Earth Sci 77:254
Manikandan S, Chidambaram C, Prasanna MV, Rakesh RG (2019) Assessment of heavy metals pollution and stable isotopic signatures in hard rock aquifers of Krishnagiri District, South India. Geosciences 9:200
Prasanna MV, Nagarajan R, Chidambaram S, Anand Kumar A, Thivya C (2017) Evaluation of hydrogeochemical characteristics and the impact of weathering in seepage water collected within the sedimentary formation. Acta Geochem 36:44–51
Acknowledgements
The authors from Tripura University thank the Head of the Department of Chemistry, Tripura University for providing necessary laboratory support to carry out the chemical analysis for the water samples. The authors are also thankful to Ms. Ninu Krishnan Modon Valappil for producing geological and spatial maps.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paul, R., Prasanna, M.V., Gantayat, R.R. et al. Groundwater quality assessment in Jirania Block, west district of Tripura, India, using hydrogeochemical fingerprints. SN Appl. Sci. 1, 1055 (2019). https://doi.org/10.1007/s42452-019-1092-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1092-1