Abstract
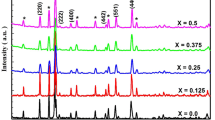
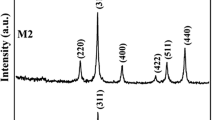
The addition of Averrhoa bilimbi (AB) extract to the Mg0.5Zn0.5Fe2O4 nanoparticles has been demonstrated to enhance the magnetic properties of the material, which was synthesized by the Coprecipitation method. XRD reveals that Mg0.5Zn0.5Fe2O4 has an average crystallite size of 8 nm and a lattice constant of 8.0554 Å; whereas the addition of Averrhoa bilimbi juice to Mg0.5Zn0.5Fe2O4 has an average crystallite size of 12 nm and a lattice constant of 8.042 Å. This small difference of 4 nm significantly impacted the spinel nanostructure. The FTIR spectrum of the AB-Mg0.5Zn0.5Fe2O4 nanoparticles has revealed two significant absorption bands; the first absorption band is attributed to the stretching vibration of the tetrahedral Mg–O and Zn–O bonds. The second absorption band is attributed to the stretching vibration of the octahedral Fe–O bonds and confirming the spinel ferrite structure. VSM shows that the coercivity of Mg0.5Zn0.5Fe2O4 soft ferrimagnetic material decreases at 600 °C when Averrhoa Bilimbi is added, while its saturation magnetization increases. Consequently, Averrhoa Bilimbi fruit acts as an excellent capping agent to enhance magnetization. These ferrite nanoparticles have been demonstrated to exhibit antibacterial activity, inhibiting the growth of harmful bacteria such as Staphylococcus aureus, Proteus vulgaris, and Escherichia coli. This is achieved by creating a magnetic field that disrupts the bacterial cell membrane, resulting in cell death. So, this material has very interesting potential applications in the biological field, such as Magnetic Resonance Imaging (MRI), Magnetic Hyperthermia Therapy, Magnetic Drug Targeting, Magnetic Separation of Cells, Magnetic Biosensors, Magnetic Filters, Magnetic Particle Imaging, Magnetic Separation of DNA, Magnetic Separation of Proteins, Magnetic Separation of Enzymes, and Magnetic Separation of Cells.








Similar content being viewed by others
Data availability
Data are confidential.
Abbreviations
- NPs:
-
Nanoparticles
- AB:
-
Averrhoa bilimbi
- FTIR:
-
Fourier Transform Infra-red Spectroscopy
- XRD:
-
X-ray diffraction
- VSM:
-
Vibrating sample magnetometer
- TV:
-
Tetrahedral voids
- OV:
-
Octahedral voids
- CFSE:
-
Crystal field stabilization energy
- RT:
-
Room temperature
- ROS:
-
Reactive oxygen species
- ABA:
-
Antibacterial activity
- MZF:
-
Mg0.5Zn0.5Fe2O4
- MZFAB:
-
Mg0.5Zn0.5Fe2O4/Averrhoa Bilimbi
References
Pradhan SK, Bid S, Gateshki M, Petkov V (2005) Microstructure characterization and cation distribution of nanocrystalline magnesium ferrite prepared by ball milling. Mater Chem Phys 93:224–230. https://doi.org/10.1016/j.matchemphys.2005.03.017
Krithiga R, Chandrasekaran G (2009) Synthesis, strucutral and optical properties of vanadium doped zinc oxide nanograins. J Cryst Growth 311:4610–4614. https://doi.org/10.1016/j.jcrysgro.2009.08.033
Elilarassi R, Chandrasekaran G (2010) Structural, optical and magnetic properties of nanoparticles of ZnO:Ni—DMS prepared by sol–gel method. Mater Chem Phys 123:450–455. https://doi.org/10.1016/j.matchemphys.2010.04.039
Rashad M (2007) Magnetic properties of nanocrystalline magnesium ferrite by co-precipitation assisted with ultrasound irradiation. J Mater Sci 42:5248–5255. https://doi.org/10.1007/s10853-006-0389-9
Elilarassi R, Chandrasekaran G (2010) Structural, optical and magnetic characterization of Cu-doped ZnO nanoparticles synthesized using solid state reaction method. J Mater Sci: Mater Electron 21:1168–1173. https://doi.org/10.1007/s10854-009-0041-y
Pradeep A, Chandrasekaran G (2006) FTIR study of Ni, Cu and Zn substituted nano-particles of MgFe2O4. Mater Lett 60:371–374. https://doi.org/10.1016/j.matlet.2005.08.053
Yue Z, Li L, Zhou J, Zhang H, Gui Z (1999) Preparation and characterization of NiCuZn ferrite nanocrystalline powders by auto-combustion of nitrate–citrate gels. Mater Sci Eng B 64:68–72. https://doi.org/10.1016/S0921-5107(99)00152-X
Sagayaraj R, Aravazhi S, Chandrasekaran G (2021) Microstructure and magnetic properties of Cu0.5Co0.3Mo0.2Fe2O4 ferrite nanoparticles synthesized by Coprecipitation method. Appl Phys A 127:502. https://doi.org/10.1007/s00339-021-04653-z
Bohra M, Alman V, Arras R (2021) Nanostructured ZnFe2O4: An exotic energy material. Nanomaterials 11:1286. https://doi.org/10.3390/nano11051286
Bahadur D (1992) Current trends in applications of magnetic ceramic materials. Bull Mater Sci 15:431–439. https://doi.org/10.1007/BF02745292
Pradeep A, Priyadharsini P, Chandrasekaran G (2011) Structural, magnetic and electrical properties of nanocrystalline zinc ferrite. J Alloy Compd 509(9):3917–3923. https://doi.org/10.1016/j.jallcom.2010.12.168
Willey RJ, Noirclerc P, Busca G (1993) Preparation and characterization of magnesium chromite and magnesium ferrite aerogels. Chem Eng Commun 123:1–16. https://doi.org/10.1080/00986449308936161
de Haart LGJ, Blasse G (1985) Photoelectrochemical properties of ferrites with the spinel structure. J Electrochem Soc 132:2933. https://doi.org/10.1149/1.2113696
Huang Y, Tang Y, Wang J, Chen Q (2006) Synthesis of MgFe2O4 nanocrystallites under mild conditions. Mater Chem Phys 97:394–397. https://doi.org/10.1016/j.matchemphys.2005.08.035
Priyadharsini P, Pradeep A, Sambasiva Rao P, Chandrasekaran G (2009) Structural, spectroscopic and magnetic study of nanocrystalline Ni–Zn ferrites. Mater Chem Phys 116(1):207–213. https://doi.org/10.1016/j.matchemphys.2009.03.011
Sivakumar M, Takami T, Ikuta H, Towata A, Yasui K, Tuziuti T, Kozuka T, Bhattacharya D, Iida Y (2006) Fabrication of zinc ferrite nanocrystals by sonochemical emulsifcation and evaporation: observation of magnetization and its relaxation at low temperature. J Phys Chem B 110:15234–15243. https://doi.org/10.1021/jp055024c
Xuan SH, Wang F, Wang Y-XJ, Yu JC, Leung KC-F (2010) Facile synthesis of size-controllable monodispersed ferrite nanospheres. J Mater Chem 20:5086–5094. https://doi.org/10.1039/c0jm00159g
Cornell RM, Schwertmann U (2003) The iron oxides: structure, properties, reactions, occurences and uses. Wiley-Blackwell, Hoboken. https://doi.org/10.1002/3527602097
Sagayaraj R, Aravazhi S, Praveen P et al (2018) Structural, morphological and magnetic characters of PVP coated ZnFe2O4 nanoparticles. J Mater Sci: Mater Electron 29:2151–2158. https://doi.org/10.1007/s10854-017-8127-4
Revathi V, Karthik K (2018) Microwave-assisted CdO-ZnO-MgO nanocomposites and its photocatalytic and antibacterial studies. J Mater Sci: Mater Electron 29:18519–18530. https://doi.org/10.1007/s10854-018-9968-1
Soberón JR, Sgariglia MA, Sampietro DA, Quiroga EN, Vattuone MA (2010) Free radical scavenging activities and inhibition of inflammatory enzymes of phenolics isolated from Tripodanthus acutifolius. J Ethnopharmacol 130(2):329–333. https://doi.org/10.1016/j.jep.2010.05.015
Halliwell B (1996) Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radic Res 25:57–74. https://doi.org/10.3109/10715769609145656
Naik MM, Naik HSB, Nagaraju G, Vinuth M, Vinu K, Rashmi SK (2018) Effect of aluminium doping on structural, optical, photocatalytic and antibacterial activity on nickel ferrite nanoparticles by sol–gel auto-combustion method. J Mater Sci: Mater Electron 29:20395–20414. https://doi.org/10.1007/s10854-018-0174-y
Karthik K, Dhanuskodi S, Gobinath C, Prabukumar S, Sivaramakrishnan S (2018) Multifunctional properties of microwave assisted CdO–NiO–ZnO mixed metal oxide nanocomposite: enhanced photocatalytic and antibacterial activities. J Mater Sci: Mater Electron 29:5459–5471. https://doi.org/10.1007/s10854-017-8513-y
Saha SK, Lee SB, Won J, Choi HY, Kim K, Yang G-M, Dayem AA, Cho S (2017) Correlation between oxidative stress, nutrition, and cancer initiation. Int J Mol Sci 18(7):1544. https://doi.org/10.3390/ijms18071544
Mannan A, Rahman MM, Habib MR, Hasan MA, Al Amin M, Saha A (2014) Comparative assessment on in vitro antioxidant activities of ethanol extracts of Averrhoa bilimbi, Gymnema sylvestre and Capsicum frutescens. Pharmacogn Res 6(1):36. https://doi.org/10.4103/0974-8490.122915
Nadeem K, Rahman S, Mumtaz M (2015) Effect of annealing on properties of Mg doped Zn-ferrite nanoparticles. Progr Nat Sci Mater Int 25(2):111–116. https://doi.org/10.1016/j.pnsc.2015.02.001
Sebastian RM, Xavier S, Mohammed EM (2015) Dielectric behavior and AC conductivity of Mg2+ doped zinc ferrite nanoparticles synthesized by sol-gel technique. Ferroelectrics 481(1):48–56. https://doi.org/10.1080/00150193.2015.1049491
John SP, Mathew J (2018) Determination of ferromagnetic, superparamagnetic and paramagnetic components of magnetization and the effect of magnesium substitution on structural, magnetic and hyperfine properties of zinc ferrite nanoparticles. J Magn Magn Mater 475:160–170. https://doi.org/10.1016/j.jmmm.2018.11.030
Tsay C-Y, Chiu Y-C, Tseng Y-K (2019) Investigation on structural, magnetic, and FMR properties for hydrothermally-synthesized magnesium-zinc ferrite nanoparticles. Physica B 570:29–34. https://doi.org/10.1016/j.physb.2019.05.037
Tatarchuk T, Myslin M, Mironyuk I, Bououdina M, Pędziwiatr A, Gargula R, Kurzydło P (2019) Synthesis, morphology, crystallite size and adsorption properties of nanostructured Mg–Zn ferrites with enhanced porous structure. J Alloys Compd. https://doi.org/10.1016/j.jallcom.2019.152945
Soumya SL, Nair BR (2016) Assessment of heavy metals in Averrhoa bilimbi and A. carambolafruit samples at two developmental stages. Environ Monit Assess 188(5):291. https://doi.org/10.1007/s10661-016-5298-z
Petrova E, Kotsikau D, Pankov V, Fahmi A (2018) Influence of synthesis methods on structural and magnetic characteristics of Mg–Zn-ferrite nanopowders. J Magn Magn Mater. https://doi.org/10.1016/j.jmmm.2018.09.128
El-Sayed HM, Ali IA, Azzam A, Sattar AA (2017) Influence of the magnetic dead layer thickness of Mg-Zn ferrites nanoparticle on their magnetic properties. J Magn Magn Mater 424:226–232. https://doi.org/10.1016/j.jmmm.2016.10.049
Ashok A, Ratnaji T, John Kennedy L, Judith Vijaya J, GnanaPragash R (2020) Magnetically recoverable Mg substituted zinc ferrite nanocatalyst for biodiesel production: process optimization, kinetic and thermodynamic analysis. Renewable Energy. https://doi.org/10.1016/j.renene.2020.08.081
Mezher SJ, Kadhim KJ, Abdulmunem OM, Mejbel MK (2019) Microwave properties of Mg–Zn ferrite deposited by the thermal evaporation technique. Vacuum. https://doi.org/10.1016/j.vacuum.2019.109114
Ghazi N, MahmoudiChenari H, Ghodsi FE (2018) Rietveld refinement, morphology analysis, optical and magnetic properties of magnesium-zinc ferrite nanofibers. J Magn Magn Mater 468:132–140. https://doi.org/10.1016/j.jmmm.2018.07.084
Liu L, Han A, Ye M, Feng W (2015) The evaluation of thermal performance of cool coatings colored with high near-infrared reflective nano-brown inorganic pigments: Magnesium doped ZnFe2O4 compounds. Sol Energy 113:48–56. https://doi.org/10.1016/j.solener.2014.12.034
Tatarchuk T, Naushad M, Tomaszewska J, Kosobucki P, Myslin M, Vasylyeva H, Ścigalski P (2020) Adsorption of Sr(II) ions and salicylic acid onto magnetic magnesium-zinc ferrites: isotherms and kinetic studies. Environ Sci Pollut Res 27(21):26681–26693. https://doi.org/10.1007/s11356-020-09043-1
Patil SN, Pawar AM, Tilekar SK, Ladgaonkar BP (2016) Investigation of magnesium substituted nano particle zinc ferrites for relative humidity sensors. Sens Actuators, A 244:35–43. https://doi.org/10.1016/j.sna.2016.04.019
Sundararajan M, Kennedy LJ, Aruldoss U, Pasha SK, Vijaya JJ, Dunn S (2015) Microwave combustion synthesis of zinc substituted nanocrystalline spinel cobalt ferrite: structural and magnetic studies. Mater Sci Semicond Process. https://doi.org/10.1016/j.mssp.2015.06.002
Torkian S, Ghasemi A, Razavi RS (2016) Structural and magnetic consequences of Mn0.6Zn0.4Fe2−x Gd x O4 ferrite. J Supercond Nov Magn 29:1617–1625. https://doi.org/10.1007/s10948-016-3458-6
Pradeep A, Priyadharsini P, Chandrasekaran G (2008) Sol–gel route of synthesis of nanoparticles of MgFe2O4 and XRD, FTIR and VSM study. J Magn Magn Mater 320(21):2774–2779. https://doi.org/10.1016/j.jmmm.2008.06.012
Rabanal ME, Varez A, Levenfeld B, Torralba JM (2003) Magnetic properties of Mg-ferrite after milling process. J Mater Process Technol 143:470–474. https://doi.org/10.1016/S0924-0136(03)00464-3
Dhir G, Uniyal P, Verma N (2014) K, Effect of particle size on multiferroism of barium-doped bismuth ferrite nanoparticles. Mater Sci Semicond Process 27:611–618. https://doi.org/10.1016/j.mssp.2014.07.041
Karthik K, Dhanuskodi S, Gobinath C, Prabukumar S, Sivaramakrishnan S (2018) Multifunctional properties of CdO nanostructures Synthesised through microwave assisted hydrothermal method. Mater Res Innov. https://doi.org/10.1080/14328917.2018.1475443
Karthik K, Dhanuskodi S, Kumar SP, Gobinath C, Sivaramakrishnan S (2017) Microwave assisted green synthesis of MgO nanorods and their antibacterial and anti-breast cancer activities. Mater Lett 206:217–220. https://doi.org/10.1016/j.matlet.2017.07.004
Prakash RS, Jayarajan D, Aravazhi S, Chandrasekaran G, Nithya R (2022) Influence of Ce3+ substitution on magnetic properties and antibacterial activity of manganese ferrite nanoparticles synthesized by coprecipitation method. Asian J Chem 34:2288–2296. https://doi.org/10.14233/ajchem.2022.23840
Prakash A, Sagayaraj R, Jayarajan D et al (2022) Influence of Ce3+ (rare earth element) on the structural, morphological, impedance, binding energy and ferrimagnetic properties of spinel ZnFe2O4 nanoparticles fabricated by the coprecipitation method: antibacterial activity. Chem Afr. https://doi.org/10.1007/s42250-022-00570-7
Sagayaraj R, Sebastian S (2023) Improved optical and magnetic properties of polycrystalline Z-type hexaferrites by co-precipitation method. Appl Phys A 129:98. https://doi.org/10.1007/s00339-022-06370-7
Acknowledgements
The St. Joseph's College of Arts and Science (Autonomous) provided the research lab and library facilities, which the authors gratefully acknowledge.
Funding
The authors did not receive any funding from any of the agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors affirm that they have no known financial or interpersonal conflicts that would have perceived to have an impact on the research presented in this study.
Ethical approval
No one who wrote this article used either human or animal subjects in their research.
Informed consent
None.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jayarajan, D., Sagayaraj, R., Silvan, S. et al. Green synthesis, Structural and Magnetic Properties of Mg0.5Zn0.5Fe2O4 Ferrite Nanoparticles by the Coprecipitation Method: Averrhoa bilimbi fruit. Chemistry Africa 6, 1875–1885 (2023). https://doi.org/10.1007/s42250-023-00615-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00615-5