Abstract
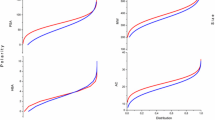
Trypanosoma cruzi causes chagas disease, a life threating disease in non-endemic and endemic regions globally, the life cycle of T. cruzi strictly depends on endogenous synthesis of sterol via 14-α-demethylase pathway. The available drugs for chagas disease treatment are currently resistance and parade unwanted side effects. Herein, molecular docking, QSAR, molecular mechanics/generalized born surface area (MM/GBSA) estimation, ADME screening, and molecular dynamics (MD) simulation were performed using Schrodinger suite to identify 14-α-demethylase protease antagonist from Ilex kudingcha. Density function theory of the hit ligands was carried out using Spartan 14 to investigate the molecular reactivity of the lead molecules. Nine (9) hit molecules were predicted as 14-α-demethylase protease inhibitors with binding energy range of − 7.632 to − 9.559 kcal/mol which was comparable to the standard drug (benznidazole = − 6.969), two lead molecules were further subjected to MD simulation over 50 ns predicting that kulactone and gallocatechin form stable interactions with vital residues at the catalytic site of the protein. DFT analysis revealed that, the hit ligands have the ability to donate and accept proton donating and accepting hence, effective as solubility and inhibitory agent and the ADME screening revealed, all the hit ligands obey Lipinski rule of five presenting them as drug candidate. The observations from this study predict kulactone and gallocatechin as putative antagonist of 14-α-demethylase protease and should be experimentally verify as a lead compound for chagas disease therapy.







Similar content being viewed by others
Data availability
The research data associated with this paper is available, it can be accessible on request from the authors.
References
Omoboyowa DA (2022) Exploring molecular docking with E-pharmacophore and QSAR models to predict potent inhibitors of 14-α-demethylase protease from Moringa spp. Pharmacol Res-Modern Chin Med 4:100147. https://doi.org/10.1016/j.prmcm.2022.100147
Lidani KCF, Andrade FA, Bavia L, Damasceno FS, Beltrame MH, Messias-Reason IJ, Sandri TL (2019) Chagas disease: from discovery to a worldwide health problem. Front Public Health 7:166. https://doi.org/10.3389/fpubh.2019.00166
Conlan J, Lal A (2015) Socioeconomic burden of foodborne parasites. In: Gajadhar AA (ed) Woodhead Publishing Series in food science, technology and nutrition, foodborne parasites in the food supply web. Woodhead Publishing, pp 75–98. https://doi.org/10.1016/B978-1-78242-332-4.00005-9
Ribeiro A, Nunes M, Teixeira M (2012) Diagnosis and management of Chagas disease and cardiomyopathy. Nat Rev Cardiol 9:576–589. https://doi.org/10.1038/nrcardio.2012109
Buckner FS (2008) Sterol 14-demethylase inhibitors for trypanosoma cruzi infections. Drug targets in kinetoplastid parasites. Springer, pp 61–80
Antas PR, Medrano-Mercado N, Torrico F, Ugarte-Fernandez R, Gómez F, Correa Oliveira R, Chaves AC, Romanha AJ, Araújo-Jorge TC (1999) Early, intermediate, and late acute stages in Chagas’ disease: a study combining anti-galactose IgG, specific serodiagnosis, and polymerase chain reaction analysis. Am J Trop Med Hyg 61(2):308–314. https://doi.org/10.4269/ajtmh.1999.61.308
Andrade DV, Gollob KJ, Dutra WO (2014) Acute chagas disease: new global challenges for an old neglected disease. PLoS Negl Trop Dis 8(7):e3010. https://doi.org/10.1371/journal.pntd.0003010
Pinheiro E, Brum-Soates L, Reis R, Cubides J (2017) Chagas disease: review of needs, neglect, and obstacles to treatment access in Latin America. Rev Soc Bras Med Trop 50(3):296–300. https://doi.org/10.1590/0037-8682-0433-2016
Lepesheva GI, Villalta F, Waterman MR (2011) Targeting Trypanosoma cruzi sterol 14α-demethylase (CYP51). Adv Parasitol 5:65–87. https://doi.org/10.1016/B978-0-12-385863-4.00004-6
Fabricant DS, Farnsworth NR (2001) The value of plants used in traditional medicine for drug discovery. Environ Health Perspect 109(Suppl 1):69–75
Li L, Xu LJ, Ma GZ, Dong YM, Peng Y, Xiao PG (2013) The large-leaved Kudingcha (Ilex latifolia Thunb and Ilex kudingcha C.J. Tseng): a traditional Chinese tea with plentiful secondary metabolites and potential biological activities. J Nat Med 67(3):425–437. https://doi.org/10.1007/s11418-013-0758-z
Sun Y, Xu W, Zhang W, Hu Q, Zeng X (2011) Optimizing the extraction of phenolic antioxidants from kudingcha made from Ilex kudingcha C.J. Tseng by using response surface methodology. Sep Sci Technol 78:311–320
Soniran O, Ngele K, Onyemeziri CA, Omoboyowa DA, Nnabude A (2018) Histopathological studies on the effects of chloroform and methanolic extracts of Ilex kudingcha in Trypanosoma brucei infected Albino Wistar Rats. Recent Adv Biol Med. 4:50–62. https://doi.org/10.18639/RABM.2018.04.735155
Omoboyowa DA (2022) Virtual screening of phyto-compounds from Blighia sapida as protein tyrosine phosphatase 1B inhibitor: a computational approach against diabetes. Chem Afr 5:1–11. https://doi.org/10.1007/s42250-022-00373-w
Omoboyowa DA, Iqbal MN, Balogun TA, Bodun DS, Fatoki JO, Oyeneyin OE (2022) Inhibitory potential of phytochemicals from Chromolaema odorata L against apoptosis signal-regulatory kinase 1: a computational model against colorectal cancer. Comput Toxicol 23:100235
Ferreira L, Dos Santos R, Oliva G, Andricopulo A (2015) Molecular docking and structure-based drug design strategies. Molecules 20(7):13384–13421. https://doi.org/10.3390/molecules200713384
Omoboyowa DA, Singh G, Fatoki JO, Oyeneyin OE (2022) Computational investiga-tion of phytochemicals from Abrus precatorius seeds as modulators of peroxisome proliferator-activated receptor gamma (PPAR γ). J Biomol Struct Dyn 40:1–16. https://doi.org/10.1080/07391102.2022.2091657
Kitchen D, Decornez H, Furr J, Bajorath J (2004) Docking and scoring in virtual screening for drug discovery: methods and applications. Nat Rev Drug Discov. 3(11): 935–949. https://www.nature.com/articles/nrd1549
Pace NJ, Weerapana E (2013) Diverse functional roles of reactive cysteines. ACS Chem Biol 8:283–296
Barford D (2004) The role of cysteine residues as redox-sensitive regulatory switches. Curr Opin Struct Biol 14:679–686
Maurias AJ, Weerapana E (2019) Reactive-cysteine profiling for drug discovery. Curr Opin Chem Biol 50:29–36
Genheden S, Ryde U (2015) The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discov 10(5):449–461
Ritchie TJ, Macdonald SJF, Peace S, Pickett SD, Luscombe CN (2013) Increasing small molecule drug developability in suboptimal chemical space. Med Chem Commun 4:673–680
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46:3–26
Margulis E, Dagan-Wiener A, Ives RS, Jaffari S, Siems K, Niv MY (2021) Intense bitterness of molecules: machine learning for expediting drug discovery. Comput Struct Biotechnol J 19:568–576
Ghose AK, Herbertz T, Hudkins RL, Dorsey BD, Mallamo JP (2012) Knowledge-based, central nervous system (CNS) lead selection and lead optimization for CNS drug discovery. ACS Chem Neurosci 3(1):50–68
Van-Breemen RB, Li Y (2005) Caco-2 cell permeability assays to measure drug absorption. Expert Opin Drug Metab Toxicol 1(2):175–185
Jin X, Luong TL, Reese N, Gaona HV, Collazo-Velez C, Vuong B, Potter JC, Sousa R, Olmeda Q, Li L, Xie J, Zhang P, Zhang G, Reichard V, Melendez SR, Marcsisin BS (2014) Pybus, comparison of MDCK-MDR1 and Caco-2 cell based permeability assays for anti-malarial drug screening and drug investigations. J Pharmacol Toxicol Methods 70(2):188–194. https://doi.org/10.1016/j.vascn.2014.08.002
Kwon S, Bae H, Jo J (2019) Comprehensive ensemble in QSAR prediction for drug discovery. BMC Bioinform 20:521. https://doi.org/10.1186/s12859-019-3135-4
Ponmary PLD, Jeya SSD (2010) QSAR study for the prediction of IC50 and logP for 5-N-acetyl-beta-D-neuraminic acid structurally similar compounds using stepwise (multivariate linear regression. Int J Chem Res 2(1):32–38
Tandon H, Chakraborty T, Suhang V (2019) A brief review on importance of DFT in drug design. Res Med Eng Sci 7(4):791–795
Uzzaman MT, Mahmud T (2020) Structural modification of aspirin to design new potential cyclooxygenase (COX-2) inhibitors. In Silico Pharmacol 8:1
Balogun TA, Iqbal MN, Saibu OA, Akintubosun MO, Lateef OM, Nneka UC, Abdullateef OT, Omoboyowa DA (2021) Discovery of potential HER2 inhibitors from Mangifera indica for the treatment of HER2-Positive breast cancer: an integrated computational approach. J Biomol Struct Dyn 39:1–12
Baell JB, Congreve M, Leeson P, Abad-Zapatero C (2013) Ask the experts: past, present and future of the rule of five. Fut Med Chem 5:745–752
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Omoboyowa, D.A., Kareem, J.A., Saibu, O.A. et al. Identification of Phyto-Compounds from Ilex kudingcha as Inhibitors of Sterol-14α-Demethylase Protease: A Computational Approach Against Chagas Disease. Chemistry Africa 6, 1335–1347 (2023). https://doi.org/10.1007/s42250-022-00565-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-022-00565-4