Key summary points
To evaluate the association of sarcopenia and low appendicular skeletal muscle mass with B-type natriuretic peptide (BNP) and its N-terminal fragment (NT-proBNP) levels in patients with heart failure.
AbstractSection FindingsSarcopenia was associated with significantly greater levels of BNP (MD: 87.76, 95% CI 20.74 – 154.78, I2 = 61%, P = 0.01) and NT-proBNP (MD: 947.45, 95% CI 98.97 – 1795.93, I2 = 35%, P = 0.03). Likewise, low appendicular skeletal muscle mass was linked to higher levels of BNP (MD: 118.95, 95% CI 46.91 – 191.00, I2 = 93%, P < 0.01) and NT-proBNP (MD: 672.01, 95% CI 383.72 – 960.30, I2 = 2%, P < 0.01).
AbstractSection MessageResearch is needed to determine whether sarcopenia may be a contributor to dysregulated plasma concentrations of natriuretic peptides.
Abstract
Aims
Sarcopenia is linked to impaired physical function and exercise tolerance. The aim of this systematic review and meta-analysis was to examine the association of sarcopenia and low appendicular skeletal muscle (ASM) with biomarkers of cardiac function, B-type natriuretic peptide (BNP) and its N-terminal fragment (NT-proBNP), in patients with heart failure (HF).
Methods and results
From inception until May 2023, a systematic literature search of observational studies was undertaken utilizing the PubMed, Web of Science, Scopus, and Cochrane Library databases. A meta-analysis employing a random-effects model was used to compute the pooled effects (CRD42023418465). Overall, 16 studies were included in this systematic review and meta-analysis. Our main analysis showed that sarcopenia in HF was linked to significantly higher levels of BNP (MD: 87.76, 95% CI 20.74–154.78, I2 = 61%, P = 0.01) and NT-proBNP (MD: 947.45, 95% CI 98.97–1795.93, I2 = 35%, P = 0.03). Similarly, low ASM was associated with significantly higher levels of BNP (MD: 118.95, 95% CI 46.91–191.00, I2 = 93%, P < 0.01) and NT-proBNP (MD: 672.01, 95% CI 383.72–960.30, I2 = 2%, P < 0.01). The quality of the included cohort studies was considered moderate, using the binary AXIS checklist and the Cochrane Tool to Assess the Risk of Bias in Cohort Studies.
Conclusions
In patients with HF, sarcopenia and reduced ASM are associated with considerably higher plasma levels of BNP and NT-proBNP. Future research is required to investigate whether sarcopenia may express dysregulated biomarkers of cardiac function.
Graphical abstract

Similar content being viewed by others
Introduction
Heart failure (HF) and sarcopenia are two common age-related conditions that often coexist in older adults [1]. Sarcopenia is defined as the loss of muscle mass, strength, and function, and is known to be associated with a variety of adverse health outcomes [2]. HF, on the other hand, is a clinical syndrome that results from structural or functional impairments of the heart and is characterized by multiple symptoms, such as breathlessness, fatigue, and fluid retention [3].
There is growing evidence to suggest that the presence of HF may accelerate the development of sarcopenia [4]. Epidemiological studies conducted in older adults have consistently shown that individuals with HF have a higher prevalence of sarcopenia compared to those without HF [5, 6]. In addition, individuals with HF tend to have greater declines in muscle mass and strength over time, which further contributes to the progression of sarcopenia [7, 8].
Nevertheless, the question of whether the presence of sarcopenia during HF accelerates HF progression remains unclear. Some studies have suggested that sarcopenia may be an independent predictor of adverse outcomes in HF, including increased hospitalization rates and mortality [9, 10]. However, the precise mechanisms underlying this association are poorly understood.
A potential biomarker that may shed light on the link between HF, sarcopenia, and adverse outcomes is B-type natriuretic peptide (BNP). BNP is a cardiac hormone produced in response to increased pressure and volume overload, which are hallmarks of HF [11]. Elevated levels of BNP are a well-established diagnostic and prognostic marker for HF, as they reflect the severity of underlying cardiac dysfunction [11, 12]. BNP levels tend to be higher in individuals with muscle wasting and sarcopenia, indicating a potential link between the two conditions [13,14,15]. Similarly, NT-proBNP, a biologically inactive derivative of BNP, is another biomarker that is commonly used to assess HF severity [12, 16]. However, the relationship between NT-proBNP and sarcopenia is less clear.
Furthermore, cardiac cachexia is a syndrome characterized by the loss of muscle mass and fat, which can occur in the presence of chronic HF [17]. Cardiac cachexia-induced weight loss may also contribute to the development of sarcopenia [18]. This loss of lean mass is often accompanied by an increase in circulating levels of BNP and NT-proBNP [17, 19, 20], highlighting the complex relationship between HF and skeletal muscle wasting, and the need to elucidate the mechanisms behind them.
Given the potential impact of sarcopenia on HF outcomes, it is important to explore potential links that could further explain the relationship between sarcopenia and HF. This systematic review and meta-analysis aims to compare the differences in BNP and NT-proBNP levels in patients with HF, with vs. without sarcopenia, and low vs. higher values of appendicular skeletal muscle mass (ASM), investigating sarcopenia as a potential contributor to aggravated HF states.
Methods
The revised 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria were followed to conduct this systematic review and meta-analysis [21]. The protocol has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42023418465).
Search strategy
From the beginning until May 2023, PubMed, Scopus, Web of Science, and Cochrane Library were searched independently by K.P and J.M. The search phrases “(heart failure OR ejection fraction) AND (sarcopeni* OR appendicular skeletal muscle OR appendicular lean mass OR skeletal muscle mass OR “ASMI” OR muscle mass)” were employed. Before submission, searches were conducted once more to find any other studies that matched our inclusion criteria.
Inclusion and exclusion criteria
The following criteria were used to determine which studies should be included: (i) baseline data from observational studies (i.e., cross-sectional, longitudinal, and case-control); (ii) patients with HF regardless of ejection fraction type; (iii) adults with a mean age 50 years old and above; and (iv) clear diagnostic criteria for sarcopenia (i.e., EWGSOP, AWGS, FNIH, or CHS). Published articles were excluded if they (i) were reviews, letters, in vivo or in vitro experiments, commentaries, or posters; (ii) were not published as a full text and in English; and (iii) included participants with mean age <50 years.
Data extraction and risk of bias
Data on the first author, publication date, country of origin, participants’ age (both with and without sarcopenia), study design, population studied, ejection fraction rate, number of participants, BNP and NT-proBNP levels, definition of sarcopenia, levels of ASM, body composition assessment tool, and reported comorbidities were all extracted independently by two authors (K.P and J.M). Two authors assessed the methodological quality of the studies using three separate tools for cross-sectional population-based studies and cohort-based studies. These checklists all appraise the validity, results, and generalizability of the studies. The tools thoroughly examined the impact of confounders in the quality of results and conclusions. The binary AXIS checklist was used to assess the quality of cross-sectional studies, consisting of 20 questions divided into (1) Introduction, (2) Methods, (3) Results, (4) Discussion, and (5) Other [22]. The risk of bias in cohort studies was assessed utilizing the Cochrane Tool to Assess Risk of Bias in Cohort Studies. Risk of bias appraisal included the assessment of bias domains such as: (1) cohort selection, (2) assessment of exposure, (3) outcome of interest absent at the start of the study, (4) adjustment of prognostic factors, (5) assessment of prognostic factors, (6) assessment of outcome, (7) adequate follow up, and (8) co-intervention similarities between groups. According to the scoring system, study quality was defined as low risk of bias, some concerns, or high risk of bias.
Statistical analysis
To determine mean differences (MDs) regarding the levels of BNP and NT-proBNP, quantitative data were handled as continuous measurements, and changes in outcomes from patients with sarcopenia and no sarcopenia were compared between groups. The method "standard deviation (SD) = width of IQR/1.35" was used to roughly calculate the missing SDs when studies reported the interquartile range (IQR). In case 95% confidence intervals (95% CIs) were available, SDs were obtained using the equation “SD= √N x (Upper limit of CI – Lower limit of CI)/3.92” [23]. The inverse-variance approach and the random-effects model were used to determine statistical significance.
The overlap of their 95% CIs and measures of Cochran's Q (Chi-square test) and I2 were used to analyze the statistical heterogeneity of outcome data across various studies. Low heterogeneity was defined as I2 of 30% to 49%, moderate heterogeneity as I2 of 50% to 74%, and high heterogeneity as I2 of 75% and above [24]. Age, sarcopenia definition, and geographic location were considered in subgroup analyses. Additionally, sensitivity analyses that discounted the impact of comorbidities and/or the significant differences in left ventricular ejection fraction (LVEF) rates between sarcopenia and no sarcopenia groups on outcome measurements, in accordance with the risk of bias of the included cohort studies, were carried out to assess the robustness of the reported statistical results. In relation to ASM, sensitivity analysis was also carried out regarding its definition (low vs. high/normal). The meta-analyses were performed utilizing the programme Review Manager (RevMan 5.4.1). Statistical significance was defined as a p value <0.05.
Results
Search results
The initial literature search provided 1626 publications. Following the exclusion of duplicates and abstracts, 25 full texts were identified as eligible for inclusion in the systematic review and meta-analysis. Of these 25 studies, six studies were dismissed due to similar cohorts [25,26,27,28,29,30] with data on participants included in more recent studies that were inserted in our research, one study had participants with a mean age below 50 years old [31], one study had insufficient data on sarcopenia and our outcomes of interest [32], and one study included patients with non-severe or no sarcopenia [33].
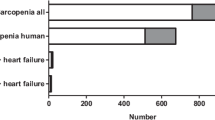
In total, 16 studies [10, 13, 20, 34,35,36,37,38,39,40,41,42,43,44,45,46] were included in this systematic review and meta-analysis exploring the relationship of BNP and NT-proBNP with sarcopenia vs. without sarcopenia and low ASM vs. higher ASM in HF (Fig. 1). Particularly, as exposures, nine studies explored this relationship using sarcopenia [34,35,36,37,38, 40, 41, 45, 46], and seven studies using low ASM [10, 13, 20, 39, 42,43,44]. Characteristics of the included studies are summarised in Tables S1 and S2.
Data transformation
Calculation of missing mean (SD) values was conducted in three studies related to sarcopenia and NT-proBNP [35, 37, 46], four studies related to sarcopenia and BNP [34, 37, 38, 40], one study related to ASM and NT-proBNP [10], and five studies in relation to ASM and BNP [13, 39, 42,43,44].
BNP levels in sarcopenia vs. no sarcopenia in HF
Our main analysis (k = 5; 372 subjects with sarcopenia and 816 subjects without sarcopenia) showed that sarcopenia was associated with significantly higher levels of BNP (MD: 87.76, 95% CI 20.74–154.78, I2 = 61%, P = 0.01) (Fig. 2).
Sensitivity analysis based on the exclusion of one study [45] for which patients with sarcopenia had a greater prevalence of hemodialysis did not alter the findings (MD: 63.03, 95% CI 23.51–102.54, I2 = 13%, P = 0.002) (Fig. S1). Identical results were displayed after the exclusion of one study [40] due to a higher risk of bias (MD: 89.16, 95% CI 15.29–163.03, I2 = 70%, P = 0.02) (Fig. S2). Conversely, excluding a study using the Ishii index to define sarcopenia [34], our analysis showed insignificant changes between groups (MD: 87.30, 95% CI − 6.41 – 181.01, I2 = 63%, P = 0.07) (Fig. S3).
NT-proBNP levels in sarcopenia vs. no sarcopenia in HF
Our main analysis (k = 5; 500 subjects with sarcopenia and 852 subjects without sarcopenia) showed that sarcopenia was associated with significantly higher levels of NT-proBNP (MD: 947.45, 95% CI 98.97 – 1795.93, I2 = 35%, P = 0.03) (Fig. 3).
Sensitivity analysis based on the exclusion of two studies [35, 36] for which patients with sarcopenia had a greater prevalence of stroke and coronary heart disease (MD: 590.23, 95% CI − 481.05–1661.50, I2 = 53%, P = 0.28) (Fig. S4). When we excluded the study with the highest risk of bias [41], people with sarcopenia exhibited statistically higher levels of NT-proBNP vs. those without sarcopenia (MD: 1178.32, 95% CI 671.53–1685.10, I2 = 0%, P < 0.01) (Fig. S5). Similarly, given that in the study by Kono et al. (2020) [41] those with sarcopenia had significantly higher ejection fraction rates compared to participants with no sarcopenia, a sensitivity analysis was also performed (MD: 1178.32, 95% CI 671.53–1685.10, I2 = 0%, P < 0.01) (Fig. S6).
BNP levels in participants with low vs. higher levels of ASM
Our main analysis (k = 5; 836 subjects with low ASM and 2190 subjects with higher ASM) showed that low ASM was associated with significantly higher levels of BNP, although an increased degree of heterogeneity was observed (MD: 118.95, 95% CI 46.91–191.00, I2 = 93%, P < 0.01) (Fig. 4).
Considering that two studies [43, 44] had very different cut-off values for low ASM, a sensitivity analysis was performed without altering our results (MD: 152.95, 95% CI 44.18–261.72, I2 = 96%, P < 0.01) (Fig. S7). In addition, sensitivity analysis based on a higher prevalence of CKD [39] in subjects with low ASM also showed similar findings (MD: 143.09, 95% CI 23.77–262.41, I2 = 89%, P = 0.02) (Fig. S8). Likewise, in relation to ASM, the exclusion of two studies that had an overall moderate risk of bias [13, 43] did not alter the findings derived from the main analysis regarding BNP levels (MD: 99.38, 95% CI 32.50–166.26, I2 = 93%, P < 0.01) (Fig. S9).
NT-proBNP levels in participants with low vs. higher levels of ASM
Our main analysis (k = 2; 382 subjects with low ASM and 425 subjects with higher ASM) showed that low ASM was associated with significantly higher levels of NT-proBNP (MD: 672.01, 95% CI 383.72–960.30, I2 = 2%, P < 0.01) (Fig. 5).
Notes on figures: modifications of forest plot scales
Considering that forest plot scales in RevMan 5.4.1 go up to 1000, we had to modify some of our plots. For example, the mean and SD values of Fig. 4 and Figs. S7-S9 were divided by 10, while Fig. 3, 5, and Fig.s S4–S6 were divided by 100.
Risk of bias of included studies
The overall quality of the included studies exploring the impact of sarcopenia was considered moderate, albeit most studies had a low risk (Table S3, Table S4). In addition, the quality of the included studies exploring the impact of low ASM was considered low (Table S5).
Discussion
In this systematic review and meta-analysis of 16 studies, we found that patients with HF and sarcopenia, and those with low ASM have higher plasma levels of BNP and NT-proBNP compared to patients without sarcopenia and higher ASM, respectively.
Although the physiological rationale underpinning the reason sarcopenia and low ASM could lead to reduced levels of cardiac function markers is limited, research has indicated that this effect may be mediated by alterations of sex steroid hormones. In particular, higher androgen concentrations have been linked to increased ASM and lower natriuretic peptide release [47,48,49], whereas oestrogens, which are associated with decreased ASM, may raise natriuretic peptide levels [50]. However, to date, evidence behind the biological processes justifying these findings is lacking, and research around this area is warranted. Furthermore, skeletal muscle growth mediators (i.e., Akt1, Follistatin-1) with potentially cardioprotective impact via regulation of endothelial cell function and blood vessel growth in skeletal muscle could partially describe this phenomenon [51,52,53]. Mechanistically, follistatin may downregulate transforming growth factor beta (TGF-β) member activity and the phosphorylation of Smad3 [54, 55]; critical promoters of muscle atrophy [56]. Nevertheless, human research has shown that increased follistatin production is linked to left ventricular adverse remodelling rather than improved left ventricular function [57]. Understanding the relationship between muscular dysfunction and heart failure may rely on fully clarifying the link between sarcopenia and elevated natriuretic peptide levels.
Moreover, individuals exhibiting substantial weight loss display elevated NT-proBNP levels compared to people with stable weight [58]. Interestingly, patients with HF and cachexia have elevated levels of BNP compared to bodyweight-stable patients [19], which have been linked to altered epicardial adipose tissue metabolism and greater regional fat thickness [59]. HF may induce the production of adiponectin and promote lipolysis through elevated levels of natriuretic peptides [60], and concomitantly, may also increase plasma levels of myostatin and proinflammatory cytokines, which are linked to muscle wasting [14, 61]. The above observations speculate that some patients with HF may have a dysregulated inflammatory, myostatin, lipolytic, and even appetite profile compared to other patients, which could in part explain our findings.
Strengths and limitations
This is the first study attempting to systematically examine the association of sarcopenia with BNP and NT-proBNP in patients with HF. Our meta-analysis employed multiple subgroup and sensitivity analyses, including controlling for comorbidities and LVEF, utilizing both sarcopenia and ASM to provide greater consistency.
Our study, however, has several limitations. We could not perform additional analyses to explore the impact of sex and whether the type of HF [with reduced (HFrEF) or preserved (HFpEF) ejection fraction] would exhibit different outcomes. Additionally, although secondary sarcopenia in patients with HF may lead to increased levels of natriuretic peptides, it is worth considering the additional impact of age-related loss of muscle mass and strength. In the included studies, sarcopenic groups were notably older than patients without sarcopenia, implying that age may be a significant contributor to our results. Albeit, ageing is linked to a higher prevalence of diastolic dysfunction, the participants included in the analysis had comparable levels of LVEF following adjustment via sensitivity analysis, highlighting that ageing could influence BNP and NT-proBNP in other ways. In addition, although both natriuretic peptides are closely related, their distribution among studies was unequal. Considering this, the non-normal distribution of biomarkers may influence the robustness of our analyses. Moreover, natriuretic peptides are some of several biomarkers of cardiac function, therefore, investigation of more biomarkers may be warranted. It is noteworthy that the studies included did not include details of the natriuretic peptide assays used, although it seems reasonable to assume these were all clinical diagnostic assays. There are several manufacturers who supply NT-proBNP and BNP assays, each with different performance characteristics. While the effect of different assay methodologies is likely to be negligible, we were not able to assess this. All results were, however, reported in standard units (ng/L or pg/ml). Added to this, it is imperative to highlight that our analytical approach deviated from utilizing raw mean (SD) values. Instead, we opted for the transformation of median (IQR) values in our analyses. Furthermore, medications could influence circulating levels of natriuretic peptides [62], however, two studies did not report details of medications [36, 45]. Finally, considering the limited observational studies, we could not extrapolate data pertinent to other individual measures of sarcopenia such as handgrip strength, gait speed, or physical performance.
Conclusions
Sarcopenia and low ASM are associated with higher plasma levels of BNP and NT-proBNP in patients with HF. In clinical practice, assessment of sarcopenia in patients with HF could potentially be complementary to such biomarkers and guide therapeutic interventions. Future research is required to investigate whether sarcopenia could lead to dysregulated biomarkers of cardiac function.
Data availability
Data is available upon request.
References
Lena A, Anker MS, Springer J (2020) Muscle wasting and sarcopenia in heart failure—the current state of science. Int J Mol Sci 21(18):6549
Cruz-Jentoft AJ et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48(1):16–31
McDonagh TA et al (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 42(36):3599–3726
Von Haehling S et al (2017) Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat Rev Cardiol 14(6):323–341
Mirzai S et al (2022) Current Approach to the Diagnosis of Sarcopenia in Heart Failure A Narrative Review on the Role of Clinical and Imaging Assessments. Circ Heart Fail 15(10):e009322
Zhang Y et al (2021) Sarcopenia in heart failure: a systematic review and meta-analysis. ESC Heart Fail 8(2):1007–1017
Reeves GR, Pandey A, Kitzman DW (2021) The other striated muscle The role of sarcopenia in older persons with heart failure. J Am Geriatr Soc. 69:1811–1814
Charkiewicz M et al (2023) Association of Chronic Heart Failure with Frailty, Malnutrition, and Sarcopenia Parameters in Older Patients—A Cross-Sectional Study in a Geriatric Ward. J Clin Med 12(6):2305
Attaway A et al (2021) Clinical impact of compound sarcopenia in hospitalized older adult patients with heart failure. J Am Geriatr Soc 69(7):1815–1825
Katano S et al (2022) Anthropometric parameters-derived estimation of muscle mass predicts all-cause mortality in heart failure patients. ESC Heart Fail 9(6):4358–4365
Yoo B-S (2014) Clinical significance of B-type natriuretic peptide in heart failure. J Lifestyle Med 4(1):34
Weber M, Hamm C (2006) Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart 92(6):843–849
Tsuchida K et al (2018) Significance of sarcopenia evaluation in acute decompensated heart failure skeletal muscle mass index versus fat-free mass index. Int Heart J 59(1):143–148
Koshikawa M et al (2020) Association between inflammation and skeletal muscle proteolysis, skeletal mass and strength in elderly heart failure patients and their prognostic implications. BMC Cardiovasc Disord 20(1):1–9
Konishi M et al (2023) Prognostic impact of upper and lower extremity muscle mass in heart failure. ESC Heart Fail 10(1):732–737
Costello-Boerrigter LC et al (2006) Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol 47(2):345–353
Hweidi IM et al (2021) Cardiac cachexia among patients with chronic heart failure a systematic review. Nurs Forum 56:916
Beltrami M, Fumagalli C, Milli M (2021) Frailty, sarcopenia and cachexia in heart failure patients: different clinical entities of the same painting. World J Cardiol 13(1):1
Janovska P et al (2020) Dysregulation of epicardial adipose tissue in cachexia due to heart failure: the role of natriuretic peptides and cardiolipin. J Cachexia, Sarcopenia Muscle 11(6):1614–1627
von Haehling S et al (2020) Muscle wasting as an independent predictor of survival in patients with chronic heart failure. J Cachexia, Sarcopenia Muscle 11(5):1242–1249
Page MJ et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 88:105906
Downes MJ et al (2016) Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 6(12):e011458
Higgins JP et al (2019) Cochrane handbook for systematic reviews of interventions. Wiley, UK
Thorlund K et al (2012) Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta-analyses. PloS One 7(7):e39471
Maeda D et al (2022) Sex differences in the prevalence and prognostic impact of physical frailty and sarcopenia among older patients with heart failure. Nutr, Metab Cardiovas Dis 32(2):365–372
da Fonseca GWP et al (2019) Sympatho-vagal imbalance is associated with sarcopenia in male patients with heart failure. Arquivos Brasileiros de Cardiologia 112:739–746
Konishi M et al (2021) Impact of sarcopenia on prognosis in patients with heart failure with reduced and preserved ejection fraction. Eur J Prevent Cardiol 28(9):1022–1029
Saito H et al (2022) Sarcopenic obesity is associated with impaired physical function and mortality in older patients with heart failure: insight from FRAGILE-HF. BMC Geriatr 22(1):556
Ohori K et al (2021) High percent body fat mass predicts lower risk of cardiac events in patients with heart failure: an explanation of the obesity paradox. BMC Geriatr 21:1–11
Gohbara M et al (2021) Skeletal muscle mass is associated with glycemic variability in patients with ST-segment elevation myocardial infarction. Heart Vessels 36:945–954
Hajahmadi M et al (2017) Muscle wasting in young patients with dilated cardiomyopathy. J Cachexia, Sarcopenia Muscle 8(4):542–548
Canteri AL et al (2019) Sarcopenia in heart failure with reduced ejection fraction. Am J Cardiovas Dis 9(6):116
Honda S et al (2022) Clinical implications of severe sarcopenia in Japanese patients with acute heart failure. Geriatr Gerontol Int 22(6):477–482
Onoue Y et al (2016) A simple sarcopenia screening test predicts future adverse events in patients with heart failure. Int J Cardiol 215:301–306
Eschalier R et al (2021) Sarcopenia in patients after an episode of acute decompensated heart failure: An underdiagnosed problem with serious impact. Clin Nutr 40(6):4490–4499
Zhao W et al (2021) The role of sarcopenia questionnaires in hospitalized patients with chronic heart failure. Aging Clin Exp Res 33:339–344
Fujimoto Y et al (2023) Prevalence and prognostic impact of the coexistence of cachexia and sarcopenia in older patients with heart failure. Int J Cardiol 381:45
Ogawa A et al (2020) Physical function and cardio-ankle vascular index in elderly heart failure patients. Int Heart J 61(4):769–775
Nishio R et al (2023) Impact of simple equation for estimating appendicular skeletal muscle mass in patients with stable coronary artery disease undergoing percutaneous coronary intervention. IJC Heart Vasc 44:101163
Fonseca G et al (2020) Discriminating sarcopenia in overweight/obese male patients with heart failure: the influence of body mass index. ESC Heart Fail 7(1):85–92
Kono Y et al (2020) The difference in determinant factor of six-minute walking distance between sarcopenic and non-sarcopenic elderly patients with heart failure. J Cardiol 75(1):42–46
Sato R et al (2020) Decreased appendicular skeletal muscle mass is associated with poor outcomes after ST-segment elevation myocardial infarction. J Atheroscler Thromb 27(12):1278–1287
Thomas E et al (2019) Bioelectrical impedance analysis of body composition and survival in patients with heart failure. Clin Cardiol 42(1):129–135
Tsuji S et al (2019) Association of serum amino acid concentration with loss of skeletal muscle mass after 1 year in cardiac rehabilitation center patients. Circ Rep 1(10):456–461
Shibasaki I et al (2022) Effect of sarcopenia on hospital stay from post cardiac surgery to discharge. IJC Heart Vasc 39:101003
Peng J et al (2023) Characteristics of the fecal microbiome and metabolome in older patients with heart failure and sarcopenia. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2023.1127041
Deng Y, Kaufman S (1993) The influence of reproductive hormones on ANF release by rat atria. Life Sci 53(9):689–696
Wu S, Weng X (1993) Regulation of atrial natriuretic peptide, thromboxane and prostaglandin production by androgen in elderly men with coronary heart disease. Chinese Med Sci J= Chung-kuo i Hsueh k’o Hsueh tsa Chih 8(4):207–209
Glisic M et al (2018) Sex steroids, sex hormone-binding globulin and levels of N-terminal pro-brain natriuretic peptide in postmenopausal women. Int J Cardiol 261:189–195
Maffei S et al (2001) Increase in circulating levels of cardiac natriuretic peptides after hormone replacement therapy in postmenopausal women. Clin Sci 101(5):447–453
Araki S et al (2012) Akt1–mediated skeletal muscle growth attenuates cardiac dysfunction and remodeling after experimental myocardial infarction. CircHeart Fail 5(1):116–125
Oshima Y et al (2008) Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation 117(24):3099–3108
Ouchi N et al (2008) Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem 283(47):32802–32811
Wang Y et al (2021) Follistatin Attenuates Myocardial Fibrosis in Diabetic Cardiomyopathy via the TGF-β–Smad3 Pathway. Front Pharmacol 12:683335
Xi Y et al (2021) Dynamic resistance exercise increases skeletal muscle-derived FSTL1 inducing cardiac angiogenesis via DIP2a–Smad2/3 in rats following myocardial infarction. J Sport Health Sci 10(5):594–603
Goodman CA et al (2013) Smad3 induces atrogin-1, inhibits mTOR and protein synthesis, and promotes muscle atrophy in vivo. Mol Endocrinol 27(11):1946–1957
Uematsu M et al (2020) Persistent myocardial production of follistatin-like 1 is associated with left ventricular adverse remodeling in patients with myocardial infarction: myocardial production of FSTL1 in AMI patients. J Cardiac Fail 26(8):733–738
Niedziela JT et al (2019) Weight loss in heart failure is associated with increased mortality only in non-obese patients without diabetes. J Cachexia, Sarcopenia Muscle 10(6):1307–1315
Nyawo TA et al (2021) A systematic review exploring the significance of measuring epicardial fat thickness in correlation to B-type natriuretic peptide levels as prognostic and diagnostic markers in patients with or at risk of heart failure. Heart Fail Rev 27:1–11
Tsukamoto O et al (2009) Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. J Am Coll Cardiol 53(22):2070–2077
Heineke J et al (2010) Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation 121(3):419–425
Troughton RW et al (2007) The effects of medications on circulating levels of cardiac natriuretic peptides. Ann Med 39(4):242–260
Acknowledgements
We would like to thank Dunhill Medical Trust for supporting this study.
Funding
This study received no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below are links to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prokopidis, K., Morwani-Mangnani, J., McDowell, G. et al. Sarcopenia is linked to higher levels of B-type natriuretic peptide and its N-terminal fragment in heart failure: a systematic review and meta-analysis. Eur Geriatr Med (2024). https://doi.org/10.1007/s41999-024-00950-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41999-024-00950-x