Abstract
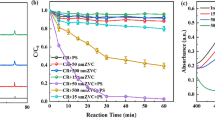
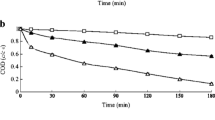
The effective treatment of dyeing wastewater has been considered as one of the challenges. The sulfate radical (SO4•−)-based technology exhibited great potential in the field of organic wastewater treatment. In this study, copper oxide was synthesized and used to activate persulfate (PS) for removing methylene blue (MB) from aqueous solution. The effects of reaction parameters and coexisting substances on this process were studied. Under the optimal conditions ([CuO] = 0.2 g/L, [PS] = 2 g/L, pH = 7.0–9.0), more than 90% of MB was degraded. Cl− had little effect on MB removal, while SO42− and HCO3− showed inhibitory effect. The activation energy of the reaction was 137.8 kJ/mol at 25 ℃ with an initial MB concentration of 10 mg/L. The mechanisms of PS activated by CuO was elucidated by radical scavenger and electron spin resonance trapping studies. The results found that sulfate radical (SO4·−) and singlet oxygen (1O2) were the primary reactive oxygen species in the CuO/PS system. The recycling experiments showed that MB removal efficiency remained more than 70% after five cycles, which exhibited good stability and high efficiency of the catalyst. The favorable degradation performance of simulated textile wastewater indicated the potential application of CuO/PS for dyeing wastewater treatment.
Article Highlights
-
CuO prepared by hydrothermal method can effectively activate PS to degraded MB.
-
The optimal degradation conditions was [CuO] = 0.2 g/L, [PS] = 2 g/L, pH = 7.0–9.0.
-
SO4·− and 1O2 were the primary reactive species in the CuO/PS system.
-
MB removal efficiency remained more than 70% after 5 cycles.







Similar content being viewed by others
Data Availability Statement
All data, models, and code generated or used during the study appear in the submitted article.
References
Ahmad M, Teel AL, Watts RJ (2013) Mechanism of persulfate activation by phenols. Environ Sci Technol 47:5864–5871
Anipsitakis GP, Dionysiou DD (2014) Radical generation by the interaction of transition metals with common oxidants. Environ Sci Technol 38:3705–3712
Chan KH, Chu W (2009) Degradation of atrazine by cobalt-mediated activation of peroxymonosulfate: Different cobalt counteranions in homogenous process and cobalt oxide catalysts in photolytic heterogeneous process. Water Res 43:2513–2521
Chen Y, Yan J, Ouyang D, Qian L, Han L, Chen M (2017) Heterogeneously catalyzed persulfate by CuMgFe layered double oxide for the degradation of phenol. Appl Catal A-Gen 538:19–26
Damasceno BS, Silva AFV, Araújo ACV (2020) Dye adsorption onto magnetic and superparamagnetic Fe3O4 nanoparticles: a detailed comparative study. J Environ Chem Eng 8:103994
Dotto J, Fagundes-Klen MR, Veit MT, Palácio SM, Bergamasco R (2019) Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J Clean Prod 208:656–665
Drzewicz P, Perez-Estrada L, Alpatova A, Martin J, Gamal EI-Din M (2012) Impact of peroxydisulfate in the presence of zero valent iron on the oxidation of cyclohexanoic acid and naphthenic acids from oil sands process-affected water. Environ Sci Technol. 46:8984–8991
Du X, Zhang Y, Hussain I, Huang S, Huang W (2017) Insight into reactive oxygen species in persulfate activation with copper oxide: activated persulfate and trace radicals. Chem Eng J 313:1023–1032
Fang G, Liu C, Gao J, Dionysiou D, Zhou D (2015) Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation. Environ Sci Technol 49:5645–5653
Feng Y, Zhou H, Liu G, Qiao J, Wang J, Lu H, Yang L, Wu Y (2012) Methylene blue adsorption onto swede rape straw (Brassica napus L.) modified by tartaric acid: equilibrium, kinetic and adsorption mechanisms. Bioresour Technol. 125:138–144
Florenza X, Solano AMS, Centellas F, Martínez-Huitle CA, Brillas E, Garcia-Segura S (2014) Degradation of the azo dye Acid Red 1 by anodic oxidation and indirect electrochemical processes based on Fenton’s reaction chemistry. Relationship between decolorization, mineralization and products. Electrochim Acta 142:276–288
Furman OS, Teel AL, Watts RJ (2010) Mechanism of base activation of persulfate. Environ Sci Technol 44:6423–6428
Ghauch A, Tuqan AM, Kibbi N (2012) Ibuprofen removal by heated persulfate in aqueous solution: a kinetics study. Chem Eng J 197:483–492
Ghauch A, Tuqan AM, Kibbi N, Geryes S (2012) Methylene blue discoloration by heated persulfate in aqueous solution. Chem Eng J 213:259–271
Guan YH, Ma J, Li XC, Fang JY, Chen LW (2011) Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/Peroxymonosulfate system. Environ Sci Technol 45:9308–9314
He Y, Li G, Wang H, Zhao J, Su H, Huang Q (2008) Effect of operating conditions on separation performance of reactive dye solution with membrane process. J Membr Sci 321:183–189
Hu P, Long M (2016) Cobalt-catalyzed sulfate radical-based advanced oxidation: a review on heterogeneous catalysts and applications. Appl Catal B 181:103–117
Hu M, Zheng S, Mi B (2016) Organic fouling of graphene oxide membranes and its implications for membrane fouling control in engineered osmosis. Environ Sci Technol 50:685–693
Huang KC, Couttenye RA, Hoag GE (2002) Kinetics of heat-assisted persulfate oxidation of methyl tert-butyl ether (MTBE). Chemosphere 49:413–420
Huang L, Zhang L, Li D, Xin Q, Jiao R, Hou X, Zhang Y, Li H (2020) Enhanced phenol degradation at near neutral pH achieved by core-shell hierarchical 4A zeolite/Fe@Cu catalyst. J Environ Chem Eng 8:103933
Ji F, Li C, Deng L (2011) Performance of CuO/Oxone system: heterogeneous catalytic oxidation of phenol at ambient conditions. Chem Eng J 178:239–243
Johnson RL, Tratnyek PG, Johnson RO (2008) Persulfate persistence under thermal activation conditions. Environ Sci Technol 42:9350–9356
Lei Y, Chen CS, Tu YJ, Huang YH, Zhang H (2015) Heterogeneous degradation of organic pollutants by persulfate activated by CuO–Fe3O4: mechanism, stability, and effects of pH and bicarbonate ions. Environ Sci Technol 49:6838–6845
Li Q, Pan F, Li W, Li D, Xu H, Xia D, Li A (2018) Enhanced adsorption of bisphenol A from aqueous solution with 2-vinylpyridine functionalized magnetic nanoparticles. Polymers 10:1136
Li H, Shan C, Pan B (2018) Fe(III)-doped g-C3N4 mediated peroxymonosulfate activation for selective degradation of phenolic compounds via high-valent iron-oxo species. Environ Sci Technol 52:2197–2205
Li Q, Wang M, Yuan X, Li D, Xu H, Sun L, Pan F, Xia D (2019) Study on the adsorption and desorption performance of magnetic resin for Congo red. Environ Technol. https://doi.org/10.1080/09593330.2019.1673830
Liang C, Su HW (2009) Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind Eng Chem Res 48:5558–5562
Liang HY, Zhang YQ, Huang SB, Hussain I (2013) Oxidative degradation of p-chloroaniline by copper oxidate activated persulfate. Chem Eng J 218:384–391
Liu C, Shih K, Sun CX, Wang F (2012) Oxidative degradation of propachlor by ferrous and copper ion activated persulfate. Sci Total Environ 416:507–512
Liu S, Huang J, Ye Y, Zhang A, Pan L, Chen X (2013) Microwave enhanced Fenton process for the removal of methylene blue from aqueous solution. Chem Eng J 215–216:586–590
Liu J, Wu P, Yang S, Rehman S, Ahmed Z, Zhu N, Dang Z, Liu Z (2020) A photo-switch for peroxydisulfate non-radical/radical activation over layered CuFe oxide: rational degradation pathway choice for pollutants. Appl Catal B 261:118232
Ma Q, Zhang H, Zhang X, Li B, Guo R, Cheng Q, Cheng X (2019) Synthesis of magnetic CuO/MnFe2O4 nanocompisite and its high activity for degradation of levofloxacin by activation of persulfate. Chem Eng J 360:848–860
Monteagudo JM, El-Taliawy H, Durán A, Caro G, Bester K (2018) Sono-activated persulfate oxidation of diclofenac: degradation, kinetics, pathway and contribution of the different radicals involved. J Hazard Mater 357:457–465
Oh WD, Dong Z, Lim TT (2016) Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: current development, challenges and prospects. Appl Catal B 194:169–201
Oliveira-Cruz FS, Nascimento MA, Puiatti GA, Oliveira AF, Mounteer AH, Lopes RP (2020) Textile effluent treatment using a fixed bed reactor using bimetallic Fe/Ni nanoparticles supported on chitosan spheres. J Environ Chem Eng 8:104133
Qi C, Liu X, Ma J, Lin C, Li X, Zhang H (2016) Activation of peroxymonosulfate by base: implications for the degradation of organic pollutants. Chemosphere 151:280–288
Qi C, Liu X, Lin C, Zhang H, Li X, Ma J (2017) Activation of peroxymonosulfate by microwave irradiation for degradation of organic contaminants. Chem Eng J 315:201–209
Qin Y, Li G, Gao Y, Zhang L, Sik-Ok Y, An T (2018) Persistent free radicals in carbon-based materials on transformation of refractory organic contaminants (rocs) in water: a critical review. Water Res 137:130–143
Saputra E, Muhammad S, Sun H, Ang H, Tadé M, Wang S (2014) Shape-controlled activation of peroxymonosulfate by single crystal α-Mn2O3 for catalytic phenol degradation in aqueous solution. Appl Catal B 154–155:246–251
Sayğılı H, Güzel F (2016) High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: process optimization, characterization and dyes adsorption. J Clean Prod 113:995–1004
Sayğılı H, Güzel F, Önal Y (2015) Conversion of grape industrial processing waste to activated carbon sorbent and its performance in cationic and anionic dyes adsorption. J Clean Prod 93:84–93
Vijayaraghavan G, Sivakumar T, Kumar AV (2011) Application of plant based coagulants for wastewater treatment. Int J Adv Eng Res Stud 1:88–92
Wacławek S, Lutze H, Grübel K, Padil V, Černík M, Dionysiou DD (2017) Chemistry of persulfates in water and wastewater treatment: a review. Chem Eng J 330:44–62
Wang J, Wang S (2018) Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem Eng J 334:1502–1517
Wang Y, Sun H, Ang HM, Tadé M, Wang S (2015) 3D-hierarchically structured MnO2 for catalytic oxidation of phenol solutions by activation of peroxymonosulfate: Structure dependence and mechanism. Appl Catal B 164:159–167
Wang J, Yang M, Liu R, Hu C, Liu H, Qu J (2019) Anaerobically-digested sludge conditioning by activated peroxymonosulfate: Significance of EDTA chelated-Fe2+. Water Res 160:454–465
Xia D, Li Y, Huang G, Yin R, An T, Li G, Zhao H, Lu A, Wong PK (2017) Activation of persulfates by natural magnetic pyrrhotite for water disinfection: efficiency, mechanisms, and stability. Water Res 112:236–247
Xie P, Ma J, Liu W, Zou J, Yue S, Li X, Wiesner M, Fang J (2015) Removal of 2-MIB and geosmin using UV/persulfate: contributions of hydroxyl and sulfate radicals. Water Res 69:223–233
Xing S, Li W, Liu B, Wu Y, Gao Y (2020) Removal of ciprofloxacin by persulfate activation with CuO: a pH dependent mechanism. Chem Eng J 382:122837
Yang S, Yang X, Shao X, Niu R, Wang L (2011) Activated carbon catalyzed persulfate oxidation of azo dye acid orange 7 at ambient temperature. J Hazard M 186:659–666
Yang Z, Dai D, Yao Y, Chen L, Liu Q, Luo L (2017) Extremely enhanced generation of reactive oxygen species for oxidation of pollutants from peroxymonosulfate induced by a supported copper oxide catalyst. Chem Eng J 322:546–555
Yang Z, Qian J, Yu A, Pan B (2019) Singlet oxygen mediated iron-based Fenton-like catalysis under nanoconfinement. P N A S 116:6659–6664
Ye Y, Jiang Z, Xu Z, Zhang X, Wang D, Lv L, Pan N (2017) Efficient removal of Cr(III)-organic complexes from water using UV/Fe(III) system: negligible Cr(VI) accumulation and mechanism. Water Res 126:172–178
Yu J, Tang L, Pang Y, Zeng G, Feng H, Zou J, Wang J, Feng C, Zhu X, Ouyang X, Tan J (2020) Hierarchical porous biochar from shrimp shell for persulfate activation: a two-electron transfer path and key impact factors. Appl Catal B 260:118160
Zhan Y, Wan X, He S, Yang Q, He Y (2018) Design of durable and efficient poly (arylene ether nitrile)/bioinspired polydopamine coated graphene oxide nanofibrous composite membrane for anionic dyes separation. Chem Eng J 333:132–145
Zhang T, Zhu H, Croué JP (2013) Production of sulfate radical from peroxymonosulfate induced by a magnetically separable CuFe2O4 spinel in water: efficiency, stability, and mechanism. Environ Sci Technol 47:2784–2791
Zhang T, Chen Y, Wang Y, Le Roux J, Yang Y, Croué J (2014) Efficient peroxydisulfate activation process not relying on sulfate radical generation for water pollutant degradation. Environ Sci Technol 48:5868–5875
Zhou L, Zheng W, Ji Y, Zhang J, Zeng C, Zhang Y, Wang Q, Yang X (2013) Ferrous-activated persulfate oxidation of arsenic(III) and diuron in aquatic system. J Hazard M 263:422–430
Acknowledgements
We gratefully acknowledge the generous support provided by the “National Natural Science Foundation of China (51908432)”, the “Support Program from the Central Government for the Local Science and Technology Development of Hubei Province (2019ZYYD068, 2018ZYYD024)”, and the “Natural Science Foundation of Hubei Province (2018CFB397),” China.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ye, Y., Wan, J., Li, Q. et al. Catalytic Oxidation of Dyeing Wastewater by Copper Oxide Activating Persulfate: Performance, Mechanism and Application. Int J Environ Res 15, 1–10 (2021). https://doi.org/10.1007/s41742-020-00296-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-020-00296-9