Abstract
Objectives
Progressive fibrosing interstitial lung disease (PF-ILD) is characterised by increased pulmonary fibrosis, lung function decline, acute exacerbations, decreased quality of life and increased mortality. Nintedanib may slow down disease progression, but long-term outcomes are unknown. We aimed to assess the cost-effectiveness of nintedanib in comparison to placebo, both on top of usual care in patients with PF-ILD.
Methods
An individual PF-ILD patient simulation model was created, using data and extrapolations from the nintedanib and placebo arms of the INBUILD trial. Clinical outcomes (mortality, exacerbations, lung transplants), economic outcomes (direct and indirect costs) and the cost-effectiveness of nintedanib over a 10-year time horizon were forecasted using the Netherlands as a case example. Disease progression was driven by lung function decline, with forced vital capacity (FVC) health states ranging from < 40 to ≥ 110 FVC of % predicted. Sensitivity and scenario analyses were performed to assess the impact of parameter assumptions on the cost-effectiveness and to test model robustness.
Results
Over a 10-year follow-up, nintedanib gained an average of 1.31 discounted life years and an average of 0.87 discounted quality-adjusted life years (QALYs), resulting in an incremental cost-effectiveness ratio (ICER) of €60,690 per QALY. Sensitivity analyses showed cost variations had a minor impact on the ICER. Results were mainly driven by mortality probabilities and disease-related utilities. Scenario analyses indicated most sensitivity to the time horizon and lung transplantation costs.
Conclusion
Long-term treatment with nintedanib could result in considerable health gains for patients with PF-ILD and can be considered cost-effective under the common willingness-to-pay threshold.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Currently, the long-term cost-effectiveness of treatment with nintedanib is unknown in patients with progressive fibrosing interstitial lung disease (PF-ILD) in the Netherlands. |
In patients with PF-ILD, treatment with nintedanib versus placebo, both on top of usual care, resulted in an incremental cost-effectiveness ratio of €60,690 per quality-adjusted life year over a 10-year time horizon. |
In our study, we showed that long-term treatment with nintedanib could result in considerable health gains for patients with PF-ILD, while being cost-effective under the common willingness-to-pay threshold in the Netherlands. |
1 Introduction and Objective
Interstitial lung diseases encompass a large and heterogeneous group of lung disorders that includes idiopathic pulmonary fibrosis (IPF), which can develop as progressive fibrosing interstitial lung disease (PF-ILD) [1,2,3]. PF-ILD is characterised by progressively increased pulmonary fibrosis, decline in lung function, acute exacerbations and increased mortality [4, 5]. Though epidemiological data for PF-ILD are limited, prevalence estimates vary between 2.2 and 20.0 per 100,000 in Europe, and 28.0 per 100,000 in the USA [6]. The annual mortality rate is expected to be comparable to IPF at around 1.4 per 100,000 [5].
The phase III INBUILD clinical trial recently evaluated treatment with nintedanib (OFEV®, Boehringer Ingelheim), an oral tyrosine kinase inhibitor, versus placebo, both on top of usual care (UC) (i.e., symptomatic treatment) in patients with PF-ILD [7]. Patients with a progressive fibrotic phenotype other than IPF were analysed as IPF and had already been studied in the INPULSIS trials [7, 8]. INBUILD showed that treatment with nintedanib resulted in a slower rate of progression. The annual rate of decline in forced vital capacity (FVC) over the 52-week period (the primary end point) was significantly lower among patients who received nintedanib (− 80.8 ml) than among patients who received placebo (− 187.8 ml; p < 0.001) [7].
Nintedanib is the first treatment approved by the European Commission for PF-ILD [9]. There are currently no other licensed treatments available for PF-ILD with European-wide approval. Immunosuppressive medicines like prednisone and azathioprine may be used off-label; however, their efficacy and safety have not been established [10]. Non-pharmaceutical treatment options include oxygen therapy, pulmonary rehabilitation, and lung transplantation [11,12,13]. Treatment with nintedanib may slow down PF-ILD progression over the course of the disease. Yet, the potential long-term clinical benefits and cost-effectiveness of treatment with nintedanib in patients with PF-ILD is unknown.
In accordance with INBUILD, patients with IPF were excluded in this cost-effectiveness analysis. In addition, the effectiveness and cost-effectiveness of nintedanib in IPF has already been analysed [8, 14, 15]. The objective of this study was to assess the cost-effectiveness of nintedanib in comparison to placebo, both on top of UC in patients with PF-ILD.
2 Methods
2.1 Study Design
An individual patient simulation model was built in Microsoft Excel. The long-term clinical effects, costs and cost-effectiveness were forecasted for treatment with nintedanib versus placebo, both on top of UC in patients with PF-ILD, using a 10-year time horizon as the follow-up period.
2.2 Model Description
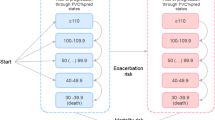
The model included 500 individual patient simulations for each arm with patient characteristics such as age (mean age 65.8 ± 9.8 years), sex (53.7% male), utilities (0.69) and percentage of predicted FVC (FVC%pred 68.99) representative for the INBUILD population. Figure 1 presents a graphical overview of the economic model detailing the PF-ILD health states (decline in predicted lung function, acute exacerbations, lung transplant and death) based on patients’ FVC ranging from < 40 to ≥ 110 FVC%pred (Appendix B, see the Electronic Supplementary Material). The survival analysis of time to discontinuation was used to separate patients into patients ‘on treatment’ or ‘off treatment’. Every generated eligible patient started at treatment allocation, each with different levels of FVC%pred and without history of acute exacerbation (randomly drawn from INBUILD). This model was informed by the cost-effectiveness analysis used in the National Institute for Health and Care Excellence (NICE) assessment for nintedanib in patients with IPF in the United Kingdom (UK) [14]. To match the nintedanib pack size usage, the model had a cycle length of 30 days (1 month) and, in each cycle, specific events could occur. Notably, half-cycle correction was applied because events and transitions can occur at any point during a cycle.
2.2.1 Lung Function Decline and Long-Term Survival
To estimate patient’s FVC%pred over time, a regression equation was derived based on a post-hoc analysis of INBUILD (Appendix B, see the Electronic Supplementary Material). In line with INBUILD, lung function decline of > 10% from baseline was considered as irreversible progression. Death occurred when a patient reached an FVC%pred value below 40%. In the model, it was assumed that each patient could transition to death from every health state [16].
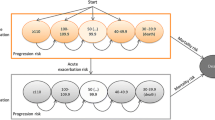
Multiple parametric extrapolations based on survival data (Kaplan–Meier curves) of INBUILD were performed including seven different survival functions; the description and choice of the extrapolations is included in Appendix B. Due to limited long-term PF-ILD data, a combined IPF dataset from the INPULSIS-ON trial including 8 years of data for treatment with nintedanib was used to visually assess the standard parametric models [17].
2.2.2 Forecasted Exacerbations, Adverse Events and Lung Transplantation
Exacerbations were defined in line with INBUILD—in short, acute, clinically significant respiratory deteriorations characterised by evidence of new, widespread alveolar abnormality [7]. Exacerbations are prognostic for mortality and morbidity in PF-ILD [7]. A regression equation was derived based on a post-hoc analysis of INBUILD (Appendix B, see the Electronic Supplementary Material).
Incorporated adverse events included diarrhoea, nausea, vomiting, alanine aminotransferase increase and decreased appetite [7]. Inclusion was based on treatment-related or treatment-emergent events, of which the incidence needed to be > 10% in the nintedanib arm and at least 1.5 times that of the control arm. In the model, patients < 65 years could undergo a lung transplant when their lung function declined by ≥ 10% compared to baseline FVC%pred [18]. After a simulated lung transplantation, ILD-related mortality rates remained in place; yet, costs and utility values resembled those for patients undergoing a lung transplant (Table C2, Appendix C) [19, 20].
2.2.3 Long-Term Cost-Effectiveness
Considering an intervention as cost-effective depends on the accepted willingness-to-pay (WTP) threshold. In this study, we chose the Netherlands as a case example where the endorsed WTP threshold for high-burden disease like PF-ILD is set at €80,000 per quality-adjusted life year (QALY) [21].
Reporting of this economic evaluation followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist (Appendix A, see the Electronic Supplementary Material) [22]. Both treatment groups (nintedanib arm versus placebo arm) were informed by INBUILD [7]. To account for future devaluations, a societal perspective with discounting rates of 4.0% for costs and 1.5% for effects and a time horizon of 10 years were applied, in line with Dutch guideline recommendations [23]. In line with the societal perspective, direct medical costs as well as direct non-medical costs (e.g. travel costs), indirect non-medical costs (e.g. work productivity losses) and indirect medical costs were included.
2.2.4 Utilities
A regression equation was used to determine patient-specific health state utilities based on EQ-5D-3L data (Electronic Supplementary Material Appendix C, description utility values modelling and Tables C1 and C2). UK preference weights were used to derive the utilities because no Dutch patients were included in INBUILD (data on file—Utilities INBUILD 2019, Clinical Trial Report, Boehringer Ingelheim). The economic model assumed utility decrements associated with treatment-related adverse events, and in the case of lung transplantation, different baseline utilities were applied. For exacerbations, a permanent drop in FVC%pred and a temporary drop in utility lasting 30 days were assumed. The permanently decreased FVC%pred would define the new utility value in the following cycle.
2.2.5 Healthcare Resource Utilisation and Associated Costs
Included costs concerned direct costs related to the intervention, societal costs, and all disease-related healthcare costs incurred until death, inflated to cost year 2019 [24]. Table C2 in Appendix C (see the Electronic Supplementary Material) presents the 1-month event probabilities for hospitalisations, emergency room (ER) visits, oxygen use and outpatient visits, per FVC%pred subgroup. All these probabilities were based on a post-hoc analysis of INBUILD. Oxygen use per patient concerned an average of 12.87 h/day and a mean of 54.51 days per year, respectively (data on file—post-hoc analysis INBUILD 2019, Boehringer Ingelheim). The monthly prescription cost of nintedanib was included in the model and distributed by dose: 70% of patients were assigned the 150 mg dose and 30% the 100 mg dose, in line with the average use in patients with IPF in the Netherlands (IQVIA in market units sales data 2020) [25, 26]. Nintedanib 60 capsules cost €2258 for 150 mg and €1683 for 100 mg per month, leading to an average daily cost of €69.52 [25]. The mean cost for grade 3 adverse events was incorporated using Dutch prices for each event [23]. The unit cost for a liver panel test was included every 3 months because elevated hepatic enzyme values were associated with nintedanib. Acute exacerbation event costs comprised €5463 and the cost for oxygen use was based on the LAN portable oxygen pump at a rate of €0.17 per hour [27, 28]. Undergoing a lung transplant comprised one-off costs of €38,214 without including other PF-ILD related costs (such as drug treatment or monitoring costs) [29]. End-of-life care such as percentages of patients that died at the intensive care unit (ICU), hospital, home or nursing home was based on Dutch data from Wuyts et al. [30].
2.3 Sensitivity Analyses
The robustness of the model results and the drivers of cost-effectiveness were explored with univariate (or one-way) sensitivity analysis (Table C3, Appendix C, see the Electronic Supplementary Material). This analysis varied the input of each probability parameter between the lowest and highest limit of their 95% confidence intervals (CIs), and for each cost parameter between the lowest and highest limits assuming a 25% variability around the mean.
Additionally, probabilistic sensitivity analysis (PSA) was performed to explore uncertainty around the model’s key variables and analyse stabilisation of the PSA outcomes. The number of iterations to achieve stabilisation was investigated. Table C3 in Appendix C presents the parameters included in the base-case setting along with their assumed distributions, and lower and upper limits.
2.4 Scenario Analyses
The following eleven scenario analyses were performed to explore alternative assumptions:
-
Time horizon: 1 (length of INBUILD), 5 and 25 years.
-
Healthcare perspective: Only taking into account the healthcare costs directly related to the intervention and all costs that occur in the years of life gained [31].
-
Utilities: Random effect excluded. It was included in the base-case analysis to better model the uncertainty in individual patient simulations.
-
Variation in drug costs: ± 20% for nintedanib. (Earlier studies and analyses have implemented the same approach [32, 33].)
-
Variation in relative dose intensity: 50% both 100 mg and 150 mg, 100% 150 mg and 100% 100 mg nintedanib.
-
Excluding end-of-life costs.
-
Excluding lung transplantation events.
-
Number of acute exacerbations: Three, over a time horizon of 10 years.
-
Gompertz distribution (parametric function for overall survival): Both nintedanib and placebo arms.
-
Loglogistic distribution (parametric function for overall survival): Both nintedanib and placebo arms.
-
Standard Weibull distribution (parametric function for overall survival): both nintedanib and placebo arms.
3 Results
An overview of seven different parametric extrapolations is shown for the overall survival in the nintedanib arm (Fig. 2). The Bayesian Weibull model was considered the best parametric distribution for overall survival based on comparability with clinical trials and observational studies with IPF (see Appendix B in the Electronic Supplementary Material for further description).
When compared to INBUILD, 97.50% of patients were alive after 6 months and 93.70% after 12 months in the model versus 98.64% and 95.31% in INBUILD, respectively, showing a difference of 1.14% for 6 months and 1.61% for 12 months. Overall, treatment with nintedanib was predicted to result in 2.87 discounted QALYs versus 2.00 discounted QALYs for placebo, both on top of UC. This led to 0.87 incremental QALYs compared with placebo over the 10-year time horizon.
The incidence of acute exacerbations with nintedanib was 3.9% per 1000 patient years versus 6.0% with UC. The average age at death was 70.88 years with nintedanib versus 69.24 years with placebo, resulting in 1.64 undiscounted incremental life years.
3.1 Cost-Effectiveness
The total costs per patient over 10 years of treatment were €91,301 for nintedanib versus €38,358 for placebo, both on top of UC. This resulted in a total incremental cost of €52,944 (discounted) over 10 years of treatment for nintedanib (Fig. 3). Treatment with nintedanib constituted 66% of the total costs per patient. Two main cost drivers in both treatment groups were patient monitoring costs (€13,102 for nintedanib vs €8931 for placebo) and the lung transplant cost, which comprised 14% of the total cost for the nintedanib group and 67% for placebo. Societal costs accounted for around €2403 for nintedanib and €1627 for placebo. The costs of acute exacerbations and for managing adverse events were minor in both groups. Together with 1.31 discounted incremental life years and 0.87 incremental QALYs (Fig. 3), this produced an incremental cost-effectiveness ratio (ICER) of €40,418 per life year gained (LYG) and an ICER of €60,690 per QALY.
3.2 Sensitivity Analysis
Figure 4 shows the effect of key parameters on the ICER per QALY. The results were most sensitive to mortality probabilities and disease-related utilities. Results were also sensitive to progression probabilities, background follow-up costs, lung transplant-related utilities and exacerbation probabilities. Other parameters, including discontinuation probabilities, societal resource use, adverse event disutilities, costs and probabilities, acute exacerbation resource use, costs for end of life and lung transplants, had a minor influence on the ICER per QALY.
The probabilistic results were stabilised with 1000 iterations (Table C4 in Appendix C, see the Electronic Supplementary Material) and are shown in Fig. 5. The PSA indicate a high probability of meeting the WTP threshold of €80,000 per QALY. The cost-effectiveness acceptability curve indicates that treatment with nintedanib has 100% probability of being cost-effective at a WTP threshold of €80,000 per QALY (Fig. 1 Appendix C).
3.3 Scenario Analyses
Scenario analyses were performed to analyse the robustness and assumptions of the economic model (Fig. 6, and in more detail in Table C5, Appendix C, see the Electronic Supplementary Material). Scenario analyses showed the model is most sensitive to the chosen time horizon and exclusion or inclusion of lung transplantation. Varying the time horizon gave ICERs of, consecutively, €2,294,659 per QALY for 1 year, €105,280 per QALY for 5 years and €55,509 per QALY for 25 years. So, the longer the time horizon, the more the ICER decreased. When lung transplantation was excluded from the model, the ICER increased to €84,926 per QALY. Varying drug cost gave ICERs of €46,882 and €74,497 per QALY, showing that the ICER was sensitive to drug cost. Also, when varying the relative dose intensity to 100% 100 mg nintedanib, this resulted in an ICER of €47,365. Varying the extrapolations to either Gompertz, Loglogistic or standard Weibull for both arms resulted in an ICER range from €57,479 to €74,254 per QALY. All other scenarios gave an ICER ranging from €56,883 to €66,401 per QALY, showing that there was limited impact on the outcome.
4 Discussion
In this study, we aimed to assess the cost-effectiveness of nintedanib in comparison to placebo, both on top of UC in patients with PF-ILD. Over a 10-year follow-up, treatment with nintedanib avoided an average of 2.1% acute exacerbations per 1000 patient years, resulted in 1.31 discounted incremental life years and 0.87 incremental QALYs, leading to an ICER of €60,690 per QALY. Sensitivity analyses showed that our analysis was generally robust. Mortality probabilities (e.g. extrapolated survival functions) and disease-related utilities had the largest impact on our value estimate. Scenario analyses showed that the model is sensitive, in particular, to the chosen time horizon and exclusion or inclusion of lung transplantation. Varying drug costs and the relative dose intensity of 100% 100 mg nintedanib also had an effect on the results. For model robustness, we varied the drug costs to both sides (± 20%). We are aware that in real life the drug costs often only decrease in a product life cycle; however, earlier studies and analyses have implemented the same approach [32, 33]. We wanted to provide a base benchmark, varying the survival estimates by including the three distributions with low Akaike information criterion (AIC) and Bayesian information criterion (BIC), and using the Gompertz distribution with the lowest survival estimates. Varying the extrapolations to either Gompertz, Loglogistic or standard Weibull distributions for both arms resulted in an ICER range from €57,479 to €74,254 per QALY. The lowest survival estimates with the Gompertz distribution resulted in an ICER of €74,254 per QALY, which is within the acceptable WTP threshold of €80,000 in the Netherlands. All other scenarios had a limited impact on the ICER.
The long-term effects and cost-effectiveness of nintedanib for the treatment of patients with PF-ILD as informed by the INBUILD trial have not been published previously in peer reviewed journals [7]. Following the main analysis of INBUILD, the effects of nintedanib were also established in five prespecified subgroup analyses: hypersensitivity pneumonitis, autoimmune ILDs, idiopathic non-specific interstitial pneumonia, unclassifiable idiopathic interstitial pneumonia, and other ILDs [34]. Although INBUILD was not powered to provide evidence for the clinical benefit of nintedanib in these specific subgroups, the effect was consistent in reducing the rate of FVC decline [3, 34]. This would suggest that nintedanib would be cost-effective for all these five subgroups in the Netherlands.
We decided to include a time horizon of 10 years based on two factors: the baseline age of the patient population in INBUILD, which was 65.8 ± 9.8 years of age, and a study by Wijsenbeek et al., in which it was estimated that the time from symptom onset to death was 61–80 months in patients who developed a non-IPF ILD progressive fibrotic phenotype [35]. Recently, the effects of nintedanib on ILD progression over the whole INBUILD trial were published. In the nintedanib (n = 332) and placebo (n = 331) groups, the proportions of patients who had ILD progression (absolute decline in FVC ≥ 10% predicted) or died were 40.4% and 54.7% in the overall population (hazard ratio 0.66 [95% CI 0.53–0.83]; p = 0.0003) over 15.6 and 16.8 months, respectively. This confirms our choice of the 10-year time horizon. We used UK preference weights to derive utilities as no Dutch patients were included in the INBUILD trial. If local Dutch utility values would have been available, we would have applied these. However, we do not expect that this would have a major impact on the results as UK values are generally considered representative for the Netherlands.
Of note, while lung transplant costs were an important driver of cost-effectiveness, the included cost of a lung transplant (€38,214) only concerned the cost for the lung transplantation itself. Costs such as research for transplant eligibility, medical support during a transplant and rehabilitation after a transplant were not included and could result in a more favourable ICER for nintedanib [36].
Sensitivity analyses showed acute exacerbations were not a major driver of the cost-effectiveness results, although the absence of effect may be driven by lack of data as acute exacerbations were rare in INBUILD and the time to first acute exacerbation was used from INBUILD to simulate this in the model. Overall, acute exacerbations meaningfully worsen the disease progression, with a concomitant negative impact on quality of life and overall survival, and a relative increase in healthcare resource use and cost [3, 37].
The cost-effectiveness of nintedanib indicated for IPF was previously peer reviewed in journals for various countries, although the evaluation of nintedanib versus placebo was only performed in the UK [14]. The comparisons used in other evaluations concerned pirfenidone or other treatments. The UK analysis indicated an ICER of £145,310 per QALY gained [14]. The outcomes differed in comparison to our evaluation; we used Dutch drug costs (which were lower), and we also used a nintedanib dose distribution in which 70% of patients were assigned the 150-mg dose and 30% were assigned 100 mg. This resulted in a lower daily average drug cost (€69.52 per day) than the 100% 150 mg applied by the UK analysis (converted with average exchange rate to €81.74 per day in accordance with exchangerate.org.uk). Our analysis, focused on PF-ILD, resulted in an incremental QALY gain of 0.87 versus the analysis for IPF that resulted in a 0.40 incremental QALY gain. Both analyses showed comparable incremental costs; therefore, the difference in incremental QALY impacted the ICER. Lastly, our analysis was performed from the Dutch societal perspective, including discount rates of 4.0% for costs and 1.5% for effects, respectively (UK, 3.5% for both), productivity costs, and the Dutch cost data for healthcare resource use, which also resulted in a lower ICER.
The base case was informed by the current usage of nintedanib of IPF patients in the Netherlands [25]. In this analysis, we included the following three scenarios: 50% both 100 mg and 150 mg, 100% 150 mg, and 100% 100 mg nintedanib. We are aware that this is not evidence based; however, we wanted to show the sensitivity to the ICER, using either 100% of 100 mg or 150 mg nintedanib and decided, therefore, to also show the sensitivity of 50% for both 100 mg and 150 mg.
A strength of this economic evaluation is that it was based on a large international clinical trial, and most of the healthcare resource utilisation data were derived from the same source, adding to the internal validity of our clinical PF-ILD progression projections. Also, the model was conceptualised and developed following international best practice guidelines [38, 39].
Although no specific Dutch patient data were used to determine the utilities, we assumed that the EQ-5D-3L values were applicable to the Dutch population. This could be a potential limitation of our economic evaluation. Another limitation was that we used long-term IPF data to address the uncertainty of long-term PF-ILD extrapolation of overall survival; however, a recent study of Brown et al. found that patients with PF-ILD who received placebo in the INBUILD trial had a clinical course similar to patients with untreated IPF [40]. The duration of follow-up for the placebo arm in the IPF trial (INPULSIS-ON) was also shorter than for the nintedanib arm, meaning that the extrapolation for the placebo arm was more uncertain than for nintedanib. As nintedanib is a new treatment for patients with PF-ILD, the treatment tolerability and adherence or discontinuation rates in real life are relatively unknown.
Real-world data and prospective studies for patients with PF-ILD, like the recent observational studies of Nasser et al. and Wijsenbeek et al. and the study of Behr et al. that was performed in patients with IPF, are necessary [35, 41, 42]. These studies are important to further identify the long-term effects, tolerability, and safety of treatment with nintedanib and the effects on disease progression, overall survival, acute exacerbations and mortality in daily practice. The outcomes of a real-world study with a more diverse patient population can further support the outcomes of this cost-effectiveness analysis.
5 Conclusion
In conclusion, long-term treatment with nintedanib could result in considerable health gains for patients with PF-ILD and can be considered cost-effective under common WTP thresholds in the Netherlands. The uncertainty around the results was mainly driven by mortality probabilities and disease-related utilities. Scenario analyses showed that the model is sensitive to the chosen time horizon, the inclusion or exclusion of lung transplantation, the lowest survival estimates applying the Gompertz distribution, and varying drug costs and relative dose intensity to 100% of 100 mg nintedanib. All other scenarios had a limited impact on the ICER. Further research is needed to confirm our model projections, in particular, the long-term trial outcomes of treatment with nintedanib for PF-ILD.
References
Travis WD, Costabel U, Hansell DM et al. An Official American Thoracic Society/European Respiratory Society Statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–48.
Kolb M, Bondue B, Pesci A, et al. Acute exacerbations of progressive-fibrosing interstitial lung diseases. Eur Respir Rev. 2018. https://doi.org/10.1183/16000617.0071-2018.
George PM, Spagnolo P, Kreuter M, et al. Progressive fibrosing interstitial lung disease: clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir Med. 2020. https://doi.org/10.1016/S2213-2600(20)30355-6.
Wells AU, Brown KK, Flaherty KR, et al. What’s in a name? That which we call IPF, by any other name would act the same. Eur Respir J. 2018. https://doi.org/10.1183/13993003.00692-2018.
Cottin V, Hirani NA, Hotchkin DL et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases, vol 27. European Respiratory Review. European Respiratory Society; 2018
Olson A, Hartmann N, Patnaik P, et al. Estimation of the prevalence of progressive fibrosing interstitial lung diseases: systematic literature review and data from a physician survey. Adv Ther. 2021. https://doi.org/10.1007/s12325-020-01578-6.
Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718–27. https://doi.org/10.1056/NEJMoa1908681.
Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–82. https://doi.org/10.1056/NEJMoa1402584.
CHMP. Ofev, INN-nintedanib [Internet]. 2020. https://www.ema.europa.eu/en/documents/overview/ofev-epar-medicines-overview_en.pdf. Accessed 19 Aug 2021.
Wong AW, Ryerson CJ, Guler SA. Progression of fibrosing interstitial lung disease. Respir Res. 2020. https://doi.org/10.1186/s12931-020-1296-3.
Visca D, Mori L, Tsipouri V, et al. Effect of ambulatory oxygen on quality of life for patients with fibrotic lung disease (AmbOx): a prospective, open-label, mixed-method, crossover randomised controlled trial. Lancet Respir Med. 2018. https://doi.org/10.1016/S2213-2600(18)30289-3.
Shaw M, Collins BF, Ho LA, et al. Rheumatoid arthritis-associated lung disease. Eur Respir Rev. 2015. https://doi.org/10.1183/09059180.00008014.
Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014—an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transpl. 2015;34:1–15. https://doi.org/10.1016/j.healun.2014.06.014.
Rinciog C, Watkins M, Chang S, et al. A cost-effectiveness analysis of nintedanib in idiopathic pulmonary fibrosis in the UK. Pharmacoeconomics. 2017. https://doi.org/10.1007/s40273-016-0480-2.
Rinciog C, Diamantopoulos A, Gentilini A, et al. Cost-effectiveness analysis of nintedanib versus pirfenidone in idiopathic pulmonary fibrosis in Belgium. PharmacoEconomics Open. 2020. https://doi.org/10.1007/s41669-019-00191-w.
National Institute for Health and Care Excellence (NICE). Diagnosis and management of suspected idiopathic pulmonary fibrosis: idiopathic pulmonary fibrosis. NICE Clinical Guideline. 2013;(June).
Lancaster L, Crestani B, Hernandez P, et al. Safety and survival data in patients with idiopathic pulmonary fibrosis treated with nintedanib: pooled data from six clinical trials. BMJ Open Respir Res. 2019;6(1):e000397.
Dutch Patient Society; Report Lung fibrosis—Lung Transplantation; 2015 [Internet]. https://www.longfibrose.nl/patient-naasten/behandelingen/longtransplantatie/. Accessed 20 Aug 2021.
Loveman E, Copley VR, Colquitt J, et al. The clinical effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: a systematic review and economic evaluation. Health Technol Assess. 2015. https://doi.org/10.3310/hta19200.
Anyanwu AC, McGuire A, Rogers CA, et al. Assessment of quality of life in lung transplantation using a simple generic tool. Thorax. 2001;56(3):218–22. https://doi.org/10.1136/thorax.56.3.218.
Dutch Healthcare Institute; Report Cost-effectiveness in practice 2015; Letter to the Minister of Health, Welfare and Sport; The Hague, the Netherlands; June 26 2015; Reference 2015071883.
Husereau D, Drummond M, Petrou S et al. Consolidated health economic evaluation reporting standards (CHEERS)-explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16(2).
Hakkaart-van Roijen L, van der Linden N, Bouwmans C et al. Cost Manual: Methodology of research for costs and reference prices for economic evaluations in health care. Zorginstituut Nederland [Internet]. 2016; 1–73. www.zorginstituutnederland.nl/publicaties/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg.
Statistics Netherlands’ database, Inflation calculation. [Internet]. https://opendata.cbs.nl/statline/#/CBS/nl/dataset/83131NED/table?fromstatweb. Accessed 15 Mar 2021.
G-Standard, Dutch treatment database (Z-index), Prices for a 30-day prescription of nintedanib 150 mg and 100 mg. March 2021.
Summary of Product Characteristics—Nintedanib September 219 [Internet]. https://www.ema.europa.eu/en/documents/product-information/ofev-epar-product-information_en.pdf. Accessed 6 Jan 2022.
van Boven JFM, Tommelein E, Boussery K, et al. Improving inhaler adherence in patients with chronic obstructive pulmonary disease: a cost-effectiveness analysis. Respir Res. 2014. https://doi.org/10.1186/1465-9921-15-66.
Dutch Lung fund brochure [Internet]. 2020. https://www.longfonds.nl/sites/default/files/commerse/webshop_pdfs/Brochure%20zuurstof%20DEF%20PDF.pdf. Accessed 20 May 2021.
Dutch Healthcare Authority; Cost for Lung transplantation [Internet]. https://zorgproducten.nza.nl/ZoekZorgproduct.aspx?psId=12&pId=65720. Accessed 15 Aug 2021.
Wuyts WA, Papiris S, Manali E, et al. The burden of progressive fibrosing interstitial lung disease: a DELPHI approach. Adv Ther. 2020;37(7):3246–64. https://doi.org/10.1007/s12325-020-01384-0 (Epub 2020 May 22. PMID: 32445186; PMCID: PMC7467418).
IJzerman MJ, de Boer A, Brouwer WBF et al. Guideline for economic evaluations in healthcare; 2016.
Westerink L, Nicolai JLJ, Samuelsen C, et al. Budget impact of sequential treatment with first-line afatinib versus first-line osimertinib in non-small-cell lung cancer patients with common EGFR mutations. Eur J Health Econ. 2020;21(6):931–43. https://doi.org/10.1007/s10198-020-01186-9 (Epub 2020 Apr 23. PMID: 32328874; PMCID: PMC7366569).
Vreman RA, Geenen JW, Knies S, et al. The application and implications of novel deterministic sensitivity analysis methods. Pharmacoeconomics. 2021;39(1):1–17. https://doi.org/10.1007/s40273-020-00979-3 (Epub 2020 Dec 14. PMID: 33313990; PMCID: PMC7790801).
Wells AU, Flaherty KR, Brown KK, et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases—subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med. 2020;8(5):453–60. https://doi.org/10.1016/S2213-2600(20)30036-9.
Wijsenbeek M, Kreuter M, Olson A, et al. Progressive fibrosing interstitial lung diseases: current practice in diagnosis and management. Curr Med Res Opin. 2019;35:2015–24.
Dutch Healthcare Authority; report Tariffs Medical Specialist care. 2019.
Song JW, Hong SB, Lim CM, et al. Acute exacerbation of idiopathic pulmonary fibrosis: Incidence, risk factors and outcome. Eur Respir J. 2011. https://doi.org/10.1183/09031936.00159709.
Caro JJ, Briggs AH, Siebert U, et al. Modeling good research practices-overview: a report of the ISPOR-SMDM Modeling Good research Practices Task Force-1. Med Decis Mak. 2012. https://doi.org/10.1177/0272989X12454577.
Kaltenthaler E, Tappenden P, Paisley S et al. Nice DSU Technical Support Document 13: identifying and reviewing evidence to inform the conceptualisation and population of cost-effectiveness models. NICE DSU Technical Support Document. 2011.
Brown KK, Martinez FJ, Walsh SLF, et al. The natural history of progressive fibrosing interstitial lung diseases. Eur Respir J. 2020. https://doi.org/10.1183/13993003.00085-2020.
Behr J, Prasse A, Wirtz H et al. Survival and course of lung function in the presence or absence of antifibrotic treatment in patients with idiopathic pulmonary fibrosis: long-term results of the INSIGHTS-IPF registry. Eur Respir J. 2020;56(2).
Nasser M, Larrieu S, Si-Mohamed S, et al. Progressive fibrosing interstitial lung disease: a clinical cohort (the PROGRESS study). Eur Respir J. 2021;57(2):2002718. https://doi.org/10.1183/13993003.02718-2020. https://doi.org/10.1183/13993003.02279-2019. PMID: 32943410; PMCID: PMC8411897
Flaherty KR, Wells AU, Cottin V, et al.; INBUILD Trial Investigators. Nintedanib in progressive interstitial lung diseases: data from the whole INBUILD trial. Eur Respir J. 2022;59(3):2004538. https://doi.org/10.1183/13993003.04538-2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Boehringer Ingelheim.
Conflict of interest
CB received grants and honoraria from various pharmaceutical companies, including other companies interested in the subject of this paper. JB received consultancy fees, honorarium and research funding from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Menarini, Novartis, Pfizer, Teva and Trudell Medical to consult, give lectures, provide advice and conduct independent research, all paid to his institution. MP received grants and honoraria from various pharmaceutical companies, including other companies interested in the subject of this paper. LW is an employee of Asc Academics. JN is an employee of Boehringer Ingelheim.
Author contributions
All authors made substantial contributions to the conception, design and analysis of the work and the data. LW drafted and JB, CB, MP and JN revised the work. All authors approved the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication (from patients/participants)
Not applicable.
Availability of data and material
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Westerink, L., Nicolai, J.L.J., Postma, M.J. et al. Cost-Effectiveness of Nintedanib for Patients with Progressive Fibrosing Interstitial Lung Disease (PF-ILD). PharmacoEconomics Open 6, 647–656 (2022). https://doi.org/10.1007/s41669-022-00354-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-022-00354-2