Abstract
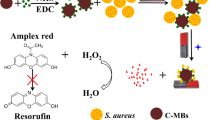
Rapid and accurate detection of pathogenic bacteria of Staphylococcus aureus (S. aureus) is of importance in the field of clinical research and practical application. Herein, we report a magnetic relaxation switching (MRS)-based strategy for detecting S. aureus in complex samples via the assistance of signal amplification system of liposome. In this design, vancomycin (Van) modified 200 nm magnetic beads (MB200-Van) and biotinylated immunoglobulin (Biotin-IgG) were employed as capture probes for S. aureus which following labelled by biotinylated liposomes loading with glucose molecule (Biotin-liposome@Glu, BLGs) based on the affinity of biotin with streptavidin. The released glucose molecules combining with glucose oxidase generated Fe3+, causing transverse relaxation time (T2) signal change. The combination of dual recognition of S. aureus, liposome assisted signal amplification, and the MRS-based assay achieved direct and specific detection of S. aureus in blood and abscess samples. The as-proposed method for S. aureus assay in this study shows potential application for infectious diagnosis in clinical.








Similar content being viewed by others
References
Fabijan AP, Lin RCY, Ho J, Maddocks S, Ben Zakour NL, Iredell JR, Khalid A, Venturini C, Chard R, Morales S, Sandaradura I, Gilbey T, Therapy WB. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat Microbiol. 2020;5(3):465–72.
Cameron D, Prazak J, Itenl M, Valente L, Grandgirard D, Leib SL, Jakob SM, Haenggi M, Que Y. Utility of nebulized bacteriophages for prophylaxis of experimental ventilator associated pneumonia due to methicillin-resistant Staphylococcus aureus. Am J Respir Crit Care Med. 2020;201:A2614.
Pressly KB, Hill E, Shah KJ. Pseudomembranous colitis secondary to methicillin-resistant Staphylococcus aureus (MRSA). BMJ Case Rep. 2016;2016: 215225.
Georgescu AM, Azamfirei L, Szalman K, Szekely E. Fatal endocarditis with methicilin-sensible Staphylococcus aureus and major complications: rhabdomyolysis, pericarditis, and intracerebral hematoma: a case report and review of the literature. Medicine. 2016;95(41): e5125.
Carestia A, Davis RP, Grosjean H, Lau MW, Jenne CN. Acetylsalicylic acid inhibits intravascular coagulation during Staphylococcus aureus-induced sepsis in mice. Blood. 2020;135(15):1281–6.
Norreslet LB, Edslev SM, Clausen ML, Flachs EM, Ebbehoj NE, Andersen PS, Agner T. Hand eczema and temporal variation of Staphylococcus aureus clonal complexes: a prospective observational study. J Am Acad Dermatol. 2021. https://doi.org/10.1016/j.jaad.2021.04.037.
Zhao XN, Yuan XM, Hu M, Zhang Y, Li LL, Zhang Q, Yuan XX, Wang WB, Liu YQ. Prevalence and characterization of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus isolated from bulk tank milk in Shandong dairy farms. Food Control. 2021;125: 107836.
Watanabe Y, Oikawa N, Hariu M, Seki M. Evaluation of agar culture plates to efficiently identify small colony variants of methicillin-resistant Staphylococcus aureus. Infect Drug Resist. 2019;12:1743–8.
Luo J, Li J, Hang Y, Yang H, Yu JP, Wei HP. Accurate detection of methicillin-resistant Staphylococcus aureus in mixtures utilizing single bacterial duplex droplet digital PCR. J Clin Microbiol. 2017;55(10):2946–55.
Wang J, Li H, Li T, Ling L. Determination of bacterial DNA based on catalytic oxidation of cysteine by G-quadruplex DNAzyme generated from asymmetric PCR: application to the colorimetric detection of Staphylococcus aureus. Microchim Acta. 2018;185(9):410.
Ottesen EA, Hong JW, Quake SR, Leadbetter JR. Microfluidic digital PCR enables multigene analysis of individual environmental bacteria. Science. 2006;314(5804):1464–7.
Alia A, Andrade MJ, Cordoba JJ, Martin I, Rodriguez A. Development of a multiplex real-time PCR to differentiate the four major Listeria monocytogenes serotypes in isolates from meat processing plants. Food Microbiol. 2020;87: 103367.
Sheng J, Tao TT, Zhu XY, Bie XM, Lv FX, Zhao HZ, Lu ZX. A multiplex PCR detection method for milk based on novel primers specific for Listeria monocytogenes 1/2a serotype. Food Control. 2018;86:183–90.
Zhao Q, Lu D, Zhang G, Zhang D, Shi X. Recent improvements in enzyme-linked immunosorbent assays based on nanomaterials. Talanta. 2021;223(1): 121722.
Seo SM, Cho IH, Jeon JW, Cho HK, Oh EG, Yu HS, Shin SB, Lee HJ, Paek SH. An ELISA-on-a-Chip biosensor system coupled with immunomagnetic separation for the detection of vibrio parahaemolyticus within a single working day. J Food Prot. 2010;73(8):1466–73.
Cao Y, Ma C, Zhu JJ. DNA Technology-assisted signal amplification strategies in electrochemiluminescence bioanalysis. J Anal Test. 2021;5(2):95–111.
Caratelli V, Fillo S, D’Amore N, Rossetto O, Pirazzini M, Moccia M, Avitabile C, Moscone D, Lista F, Arduini F. Paper-based electrochemical peptide sensor for on-site detection of botulinum neurotoxin serotype A and C. Biosens Bioelectron. 2021;183: 113210.
Geleta GS, Zhao Z, Wang ZX. Electrochemical biosensors for detecting microbial toxins by graphene-based nanocomposites. J Anal Test. 2018;2(1):20–5.
Si Y, Grazon C, Clavier G, Rieger J, Tian YY, Audibert JF, Sclavi B, Meallet-Renault R. Fluorescent copolymers for bacterial bioimaging and viability detection. ACS Sens. 2020;5(9):2843–51.
Gu GY, Wang X, Zhou HL, Liu BL. Progresses of magnetic relaxation switch sensor in medical diagnosis and food safety analysis. Chin J Anal Chem. 2018;46(8):1161–8.
Zhao Y, Li YX, Jiang K, Wang J, White WL, Yang SP, Lu J. Rapid detection of Listeria monocytogenes in food by biofunctionalized magnetic nanoparticle based on nuclear magnetic resonance. Food Control. 2017;71:110–6.
Hu YY, Guo X, Wang H, Luo Q, Song Y, Song EQ. Magnetic-separation-assisted magnetic relaxation switching assay for mercury ion based on the concentration change of oligonucleotide-functionalized magnetic nanoparticle. ACS Appl Bio Mater. 2020;3(5):2651–7.
Silva RN, Vijayan AN, Westbrook E, Yu Z, Zhang P. Nanoparticle assisted nuclear relaxation-based oligonucleotide detection. Anal Chim Acta. 2019;1062:118–23.
Lee DY, Kang S, Lee Y, Kim JY, Yoo D, Jung W, Lee S, Jeong YY, Lee K, Jon S. PEGylated bilirubin-coated iron oxide nanoparticles as a biosensor for magnetic relaxation switching-based ROS detection in whole blood. Theranostics. 2020;10(5):1997–2007.
Lee H, Shin TH, Cheon J, Weissleder R. Recent developments in magnetic diagnostic systems. Chem Rev. 2015;115(19):10690–724.
Min C, Shao HL, Liong M, Yoon TJ, Weissleder R, Lee H. Mechanism of magnetic relaxation switching sensing. ACS Nano. 2012;6(8):6821–8.
Chen YP, Zou MQ, Qi C, Xie MX, Wang DN, Wang YF, Xue Q, Li JF, Chen Y. Immunosensor based on magnetic relaxation switch and biotin-streptavidin system for the detection of Kanamycin in milk. Biosens Bioelectron. 2013;39(1):112–7.
Perez JM, O’Loughin T, Simeone FJ, Weissleder R, Josephson L. DNA-based magnetic nanoparticle assembly acts as a magnetic relaxation nanoswitch allowing screening of DNA-cleaving agents. J Am Chem Soc. 2002;124(12):2856–7.
Perez JM, Josephson L, O’Loughlin T, Hogemann D, Weissleder R. Magnetic relaxation switches capable of sensing molecular interactions. Nat Biotechnol. 2002;20(8):816–20.
Sobczak-Kupiec A, Venkatesan J, AlAnezi AA, Walczyk D, Farooqi A, Malina D, Hosseini SH, Tyliszczak B. Magnetic nanomaterials and sensors for biological detection. Nanomedicine. 2016;12(8):2459–73.
Zhang Y, Yang H, Zhou ZG, Huang K, Yang SP, Han G. Recent advances on magnetic relaxation switching assay-based nanosensors. Bioconjugate Chem. 2017;28(4):869–79.
Yin BF, Wang Y, Dong ML, Wu J, Ran B, Xie MX, Joo SW, Chen YP. One-step multiplexed detection of foodborne pathogens: Combining a quantum dot-mediated reverse assaying strategy and magnetic separation. Biosens Bioelectron. 2016;86:996–1002.
Dong M, Zheng W, Chen Y, Ran B, Qian Z, Jiang X. Cu-T1 sensor for versatile analysis. Anal Chem. 2018;90(4):2833–8.
Hu YY, Guo X, Gu PL, Luo Q, Song Y, Song EQ. Mn2+-mediated magnetic relaxation switching for direct assay of ctDNA in whole blood via exonuclease III assisted amplification. Sens Actuators B. 2021;330:129340–6.
Ganganboina AB, Chowdhury AD, Khoris IM, Nasrin F, Takemura K, Hara T, Abe F, Suzuki T, Park EY. Dual modality sensor using liposome-based signal amplification technique for ultrasensitive norovirus detection. Biosens Bioelectron. 2020;157: 112169.
Zhang JL, Gao Y, Zhang XC, Feng QS, Zhan CX, Song JL, Zhang WH, Song WB. “Dual Signal-On” split-type aptasensor for TNF-alpha: integrating MQDs/ZIF-8@ZnO NR arrays with MB-Liposome-Mediated signal amplification. Anal Chem. 2021;93(19):7242–9.
Zhang Y, Xiao JY, Zhu Y, Tian LJ, Wang WK, Zhu TT, Li WW, Yu HQ. Fluorescence sensor based on biosynthetic CdSe/CdS quantum dots and liposome carrier signal amplification for mercury detection. Anal Chem. 2020;92(5):3990–7.
Lin Y, Zhou Q, Tang D. Dopamine-loaded liposomes for in-situ amplified photoelectrochemical immunoassay of AFB1 to enhance photocurrent of Mn2+-Doped Zn3(OH)2V2O7 nanobelts. Anal Chem. 2017;89(21):11803–10.
Wu L, Zhou M, Liu C, Chen XQ, Chen YP. Double-enzymes-mediated Fe2+/Fe3+ conversion as magnetic relaxation switch for pesticide residues sensing. J Hazard Mater. 2021;403: 123619.
Gore JC, Kang YS, Schulz RJ. Measurement of radiation-dose distributions by nuclear magnetic-resonance (NMR) imaging. Phys Med Biol. 1984;29(10):1189–97.
Li LY, Gu PL, Hao MQ, Xiang XL, Feng YT, Zhu XK, Song Y, Song EQ. Bacteria-targeted MRI probe-based imaging bacterial infection and monitoring antimicrobial therapy in vivo. Small. 2021;17(44):2103627.
Zhang Y, Shi SY, Xing JJ, Tan WQ, Zhang CG, Zhang L, Yuan H, Zhang MM, Qiao JJ. A novel colorimetric sensing platform for the detection of S. aureus with high sensitivity and specificity. RSC Adv. 2019;9(58):33589–95.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22174116, 21974110), and Chongqing Science Funds for Distinguished Young Scientists (cstc2021jcyj-jqx0024), and the Innovation Research Group at higher Education Institutions in Chongqing, Chongqing Education Committee (CXQT21006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Guo, X., Deng, XC., Zhang, YQ. et al. Fe2+/Fe3+ Conversation-Mediated Magnetic Relaxation Switching for Detecting Staphylococcus Aureus in Blood and Abscess via Liposome Assisted Amplification. J. Anal. Test. 6, 111–119 (2022). https://doi.org/10.1007/s41664-022-00227-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-022-00227-x