Abstract
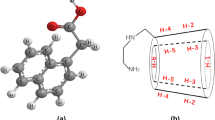
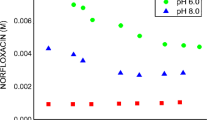
Naproxen–β-cyclodextrin inclusion complex was prepared by the freeze-drying method and analyzed by IR, UV spectroscopy, X-ray diffractometry and differential scanning calorimetry. Phase solubility, dissolution and inclusion rate of the complex were determined. The phase solubility diagram was of the A L type, which revealed that the molar ratio of naproxen to β-cyclodextrin was 1:1. The solubility of naproxen significantly increased with increasing β-cyclodextrin concentration. The apparent stability constant, K c, was calculated from the slope and intercept of the A L solubility diagram as 2376/M. The total released amount and released time of naproxen from the inclusion complex were better than that of the intact naproxen.







Similar content being viewed by others
References
Pedersen M, Bjerregaard S, Jacobsen J, et al. An econazole β-cyclodextrin inclusion complex: an unusual dissolution rate, supersaturation, and biological efficacy example. Int J Pharm. 1998;165:57–68.
Pedersen M, Edelsten M, Nielsen VF, et al. Formation and antimycotic effect of cyclodextrin inclusion complexes of econazole and miconazole. Int J Pharm. 1993;90:247–54.
Szejtli J. Cyclodextrins and their inclusion complexes. Budapest: AkademiaiKiado; 1982. p. 3–25.
Zheng Y, Haworth IS, Zuo Z, et al. Physicochemical and structural characterization of quercetin-β-cyclodextrin complexes. J Pharm Sci. 2005;94:1079–89.
Kou W, Cai C, Xu S, et al. In vitro and in vivo evaluation of novel immediate release carbamazepine tablets: complexation with hydroxypropyl-β-cyclodextrin in the presence of HPMC. Int J Pharm. 2011;409:75–80.
Zoeller T, Dressman JB, Klein S. Application of a ternary HP-β-CD-complex approach to improve the dissolution performance of a poorly soluble weak acid under biorelevant conditions. Int J Pharm. 2012;430:176–83.
Andersen FM, Bundgaard H. The influence of cyclodextrin complexation on the stability of betamethasone-17-valerate. Int J Pharm. 1984;20:155–62.
Wang Z, Deng Y, Sun S, et al. Preparation of hydrophobic drugs cyclodextrin complex by lyophilization monophase solution. Drug Dev Ind Pharm. 2006;32:73–83.
Guan TZ, Wen JW, Li GL. Study on the inclusion constant of β-CD pirooxcam by phase solubility method. Chin Chem World. 2003;4:178–80.
Guo XL, Yang Y, Zhao GY. Study on vitamin K3-cyclodextrin inclusion complex and analytical application. Spectrom Chim Acta Part A. 2003;59:3379–86.
Francesco T, Marco Z, Giovanni C. Thermal degradation of cyclodextrins. Polym Degrad Stabil. 2000;69:373–9.
Erden N, Çelebi N. A study of the inclusion complex of naproxen with β-cyclodextrin. Int J Pharm. 1988;48:83–9.
Nakajima T, Sunagawa M, Hirohashi T, et al. Studies of cyclodextrin inclusion complexes. I. complex between cyclodextrins and bencyclane in aqueous solution. Chem Pharm Bull. 1984;32:383–400.
Saenger W. Cyclodextrin inclusion compounds in research and industry. Angew Chem Int Ed. 1980;19:344–62.
Moustafa MF, Dierk K, Hipler UC. Antimycotic influence of β-cyclodextrin complexes—in vitro measurements using laser nephelometry in microtiter plates. Int J Pharm. 2006;311:113–21.
Uekama K, Fujinaga T, Hirayama F, et al. Improvement of the oral bioavailability of digitalis glycosides by cyclodextrin complexation. J Pharm Sci. 1983;72:1338–41.
Nicolazzi C, Abdou S, Collomb J, et al. Effect of the complexation with cyclodextrins on the in vitro antiviral activity of ganciclovir against human cytomegalovirus. Bioorg Med Chem. 2001;9:275–82.
Uekama K, Hirayama F, Irie T. Cyclodextrin drug carrier systems. Chem Rev. 1998;98:2045–76.
Kishore G, Shyale S, Srikanth K, et al. Development and evaluation of colon targeted tablets of praziquantel and its β-cyclodextrin complex to treat schistosomiasis. J Pharm Sci Technol. 2010;2:269–75.
Uekama K, Otagiri M. Cyclodextrins in drug carrier systems. Drug Carr Syst. 1987;3:1–40.
Sevelius MH, Runkel R, Segre E, et al. Bioavailability of naproxen sodium and its relationship to clinical analgesic effects. Br J Clin Pharmacol. 1980;10:259–63.
Higuchi T, Connors KA. Phase-solubility techniques. Adv Anal Inst. 1965;4:117–50.
Connors KA, Mollica JA Jr. Theoretical analysis of comparative studies of complex formation. J Pharm Sci. 1966;55:772–80.
Grandelli HE, Stickle B, Whittington A, Kiran E. Inclusion complex formation of β-cyclodextrin and naproxen: a study on exothermic complex formation by differential scanning calorimetry. J Incl Phenom Macrocycl Chem. 2013;77:269–77.
Liu FY, Kildsig DO, Mitra AK. Beta-cyclodextrin/steroid complexation: effect of steroid structure on association equilibria. Pharm Res. 1990;7:869–73.
Acknowledgements
This work was financially supported by the Guangdong Province Science and Technology Program Foundation (No. 2013B040200029), 2016 National Drug Evaluation Special Foundation of China and the National Natural Science Foundation of China (21375027, 21335002, and 21427806).
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Jin, G., Chen, H. Facile Preparation and Extensive Characterization of Naproxen–β-Cyclodextrin Inclusion Complex. J. Anal. Test. 1, 19 (2017). https://doi.org/10.1007/s41664-017-0021-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41664-017-0021-9