Abstract
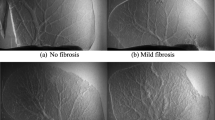
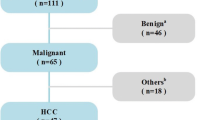
The visualization and data mining of tumor multidimensional information may play a major role in the analysis of the growth, metastasis, and microenvironmental changes of tumors while challenging traditional imaging and data processing techniques. In this study, a general trans-scale and multi-modality measurement method was developed for the quantitative diagnosis of hepatocellular carcinoma (HCC) using a combination of propagation-based phase-contrast computed tomography (PPCT), scanning transmission soft X-ray microscopy (STXM), and Fourier transform infrared micro-spectroscopy (FTIR). Our experimental results reveal the trans-scale micro-morphological HCC pathology and facilitate quantitative data analysis and comprehensive assessment. These results include some visualization features of PPCT-based tissue microenvironments, STXM-based cellular fine structures, and FTIR-based bio-macromolecular spectral characteristics during HCC tumor differentiation and proliferation. The proposed method provides multidimensional feature data support for constructing a high-accuracy machine learning algorithm based on a gray-level histogram, gray-gradient co-occurrence matrix, gray-level co-occurrence matrix, and back-propagation neural network model. Multi-dimensional information analysis and diagnosis revealed the morphological pathways of HCC pathological evolution and we explored the relationships between HCC-related feature changes in inflammatory microenvironments, cellular metabolism, and the stretching vibration peaks of biomolecules of lipids, proteins, and nucleic acids. Therefore, the proposed methodology has strong potential for the visualization of complex tumors and assessing the risks of tumor differentiation and metastasis.









Similar content being viewed by others
References
H. Liu, X. Ji, Y. Ma et al., Quantitative characterization and diagnosis via hard X-ray phase- contrast microtomography. Micro. Res. Tech. 81, 1173 (2018). https://doi.org/10.1002/jemt.23114
P. Wang, Z. Chen, D. Xing et al., Multi-parameter characterization of atherosclerotic plaques based on optical coherence tomography, photoacoustic and viscoelasticity imaging. Opt. Express 28, 13761 (2020). https://doi.org/10.1364/OE.390874
X. Ji, H. Liu, Y. Xing et al., Quantitative evaluation on 3D fetus morphology via X-ray grating based imaging technique. Int. J. Imaging Syst. Technol. 29, 677 (2019). https://doi.org/10.1002/ima.22354
H. Tan, D. Wang, R. Li et al., A robust method for high-precision quantification of the complex three-dimensional vasculatures acquired by X-ray microtomography. J. Synchrotron Radiat. 23, 1216 (2016). https://doi.org/10.1107/S1600577516011498
Y. Zhao, S. Yang, Y. Wang et al., In vivo blood viscosity characterization based on frequency-resolved photoacoustic measurement. Appl. Phys. Lett. 113, 143703 (2018). https://doi.org/10.1063/1.5039538
C. Kamezawa, T. Numano, Y. Kawabata et al., X-ray elastography by visualizing propagating shear waves. Appl. Phys. Express (2020). https://doi.org/10.35848/1882-0786/ab7e06
S. Yao, J. Fan, Z. Chen et al., Three-dimensional ultrastructural imaging reveals the nanoscale architecture of mammalian cells. IUCrJ 5, 141–149 (2018). https://doi.org/10.1107/S2052252517017912
J.Z. Hu, T.D. Wu, Z. Lei et al., Visualization of microvasculature by x-ray in-line phase contrast imaging in rat spinal cord. Phys. Med. Biol. 57, N55 (2012). https://doi.org/10.1088/0031-9155/57/5/N55
F. Wang, P. Zhou, K. Li et al., Sensitive imaging of intact micro-vessels in vivo with synchrotron radiation. IUCrJ 7, 793 (2020). https://doi.org/10.1107/S2052252520008234
G. Wei, Y. Liu, X. Ji et al., Micro-morphological feature visualization auto-classification. Cancer Med. 10, 2317 (2020). https://doi.org/10.1002/cam4.3796
J. Xiu, Y. Liu, B. Wang et al., Quantitative toxicological study of dose-dependent arsenic-induced cells via synchrotron-based STXM and FTIR measurement. Analyst 145, 4560 (2021). https://doi.org/10.1039/d0an00346h
Q. Yang, B. Deng, G. Du et al., X-ray fluorescence computed tomography with absorption correction for biomedical samples. X-Ray Spectrom. 43, 278 (2014). https://doi.org/10.1002/xrs.2550
N.S. Farrag, A.M. Amin, Preliminary evaluation of the radiotherapeutic efficacy of 131I-atorvastatin in rats with hepatocellular carcinoma. Nucl. Sci. Tech. 31(11), 106 (2020). https://doi.org/10.1007/s41365-020-00819-1
Y. Yin, K. Luo, Z. Xie et al., An incompatibility problem and solution of multimodal image-guided radiotherapy based on domestic equipment. Nucl. Tech. 44, 030301 (2021). https://doi.org/10.11889/j.0253-3219.2021.hjs.44.030301 (in Chinese)
Y. Zhou, X. Gao, C. Liu et al., The elements and specification of 18F-FDG PET-CT and PET-MR tumor imaging report in Shanghai. Nucl. Tech. 44, 010001 (2021). https://doi.org/10.11889/j.0253-3219.2021.hjs.44.010001 (in Chinese)
Y. Yang, N. Li, Bioinformatic analysis of differentially expressed genes in tumor-associated fibroblasts after ionizing radiation. J. Radiat. Res. Radiat. Process. 38(1), 32–41 (2020). https://doi.org/10.11889/j.1000-3436.2020.rrj.38.010302 (in Chinese)
B. Li, K. Hu, X. Li, Automated radiosynthesis and preliminary Micro-PET/CT imaging of [18F] AlF-NOTA-P-L-Lys6-GnRH. Nucl. Tech. 42, 080303 (2019). https://doi.org/10.11889/j.0253-3219.2019.hjs.42.080303 (in Chinese)
J. Chen, Y. Fu, D.S. Day et al., VEGF amplifies transcription through ETS1 acetylation to enable angiogenesis. Nat. Commun. 8, 1 (2018). https://doi.org/10.1038/s41467-017-00405-x
Y. Fu, H. Peng, X. Zhang et al., Assessment of fibrotic tissue and microvascular architecture by in-line phase-contrast imaging in a mouse model of liver fibrosis. Eur. Radiol. 26, 2947 (2016). https://doi.org/10.1007/s00330-015-4173-6
J.H. Duan, C.H. Hu, H. Chen et al., High-resolution micro-CT for morphologic and quantitative assessment of the sinusoid in human cavernous hemangioma of the liver. PLoS ONE 8, e53507 (2013). https://doi.org/10.1371/journal.pone.0053507
Y. Cao, Y. Zhang, X. Yin et al., 3D visualization of the lumbar facet joint after degeneration using propagation phase contrast micro-tomography. Sci. Rep. 6, 1 (2016). https://doi.org/10.1038/srep21838
H. Liu, X. Ji, L. Sun et al., Visualization and pathological characteristics of hepatic alveolar echinococcosis with synchrotron-based X-ray phase sensitive micro-tomography. Sci. Rep. 6, 1 (2016). https://doi.org/10.1038/srep38085
J. Hu, T. Wu, L. Zeng et al., Visualization of microvasculature by X-ray in-line phase contrast imaging in rat spinal cord. Phys. Med. Biol. 57, N55 (2012). https://doi.org/10.1088/0031-9155/57/5/N55
P. Coan, A. Bravin, G. Tromba et al., Phase-contrast X-ray imaging of the breast: recent developments towards clinics. J. Phys. D Appl. Phys. 46, 494007 (2013). https://doi.org/10.1088/0022-3727/46/49/494007
A. Bravin, J. Keyrilainen, M. Fernandez et al., High-resolution CT by diffraction-enhanced x-ray imaging: mapping of breast tissue samples and comparison with their histo-pathology. Phys. Med. Biol. 52, 2197 (2007). https://doi.org/10.1088/0031-9155/52/8/011
H.Q. Liu, C.S. Zhang, X.X. Fan et al., Robust phase-retrieval-based X-ray tomography for morphological assessment of early hepatic echinococcosis infection in rats. PLoS ONE 12, e0183396 (2017). https://doi.org/10.1371/journal.pone.0183396
B. Chen, G. Feng, B. He et al., Silole-based red fluorescent organic dots for bright two-photon fluorescence in vitro cell and in vivo blood vessel imaging. Small 12, 782 (2016). https://doi.org/10.1002/smll.201502822
C.P. Wang, Z.J. Xu, H.G. Liu et al., Soft X-ray ptychography method at SSRF. Nucl. Sci. Technol. 28, 74 (2017). https://doi.org/10.1007/s41365-017-0227-6
T. Ji, Y. Tong, H. Zhu et al., The status of the first infrared beamline at Shanghai synchrotron radiation facility. Nucl. Instrum. Methods. A 788, 116 (2015). https://doi.org/10.1016/j.nima.2015.03.080
P. Jovana, V. Danijela, R. Tatjana et al., Gray-level co-occurrence matrix analysis of chromatin architecture in periportal and perivenous hepatocytes. Histochem. Cell Biol. 151, 75 (2019). https://doi.org/10.1007/s00418-018-1714-5
H.Q. Liu, T.Q. Xiao, H.L. Xie et al., Nondestructive material characterization of meteorites with synchrotron-based high energy x-ray phase micro-computed tomography. J. Phys. D Appl. Phys. 50, 055301 (2017). https://doi.org/10.1088/1361-6463/aa4f31
C.P. Wang, Z.J. Xu, H.G. Liu et al., Background noise removal in x-ray ptychography. Appl. Optics 56, 2099 (2017). https://doi.org/10.1364/AO.56.002099
P.D. Kumar, Feature extraction and selection of kidney ultrasound images using GLCM and PCA. Procedia Comput. Sci. 167, 1722 (2020). https://doi.org/10.1016/j.procs.2020.03.382
T. Vujasinovic, J. Pribic, K. Kanjer et al., Gray-level co-occurrence matrix texture analysis of breast tumor images in prognosis of distant metastasis risk. Microsc. Microanal. 21, 646 (2015). https://doi.org/10.1017/S1431927615000379
S. Kaminskyj, K. Jilkine, A. Szeghalmi et al., High spatial resolution analysis of fungal cell biochemistry—bridging the analytical gap using synchrotron FTIR spectromicroscopy. FEMS Microbiol. Lett. 284, 1 (2008). https://doi.org/10.1111/j.1574-6968.2008.01162.x
H.Q. Liu, F. Lin, J. Lin et al., Phase-retrieval-based synchrotron X-ray micro-tomography for 3D structural characterization and quantitative analysis of agalloch eaglewood. Wood Sci. Technol. 52, 839 (2018). https://doi.org/10.1007/s00226-018-1004-3
M. Fernandez, J. Keyrilainen, R. Serimaa et al., Human breast cancer in vitro: matching histo-pathology with small-angle x-ray scattering and diffraction enhanced x-ray imaging. Phys. Med. Biol. 50, 2991 (2015). https://doi.org/10.1088/0031-9155/50/13/002
H.G. Liu, Z.J. Xu, C.P. Wang et al., Soft X-ray spectro-ptychography for air pollution particulates. Microsc. Microanal. 24, 44 (2018). https://doi.org/10.1017/S1431927618012655
H.G. Liu, J.F. Cao, Y. Wang et al., Soft x-ray spectroscopic end-station at beamline 08U1A of Shanghai Synchrotron Radiation Facility. Rev. Sci. Instrum. 90, 043103 (2019). https://doi.org/10.1063/1.5080760
Z. Liu, Y. Tang, F. Chen et al., Synchrotron FTIR micro-spectroscopy reveals early adipo-genic differentiation of human mesenchymal stem cells at single-cell level. Biochem. Biophys. Res. Commun. 478, 1286 (2016). https://doi.org/10.1016/j.bbrc.2016.08.112
S. Lang, P. Schachtschneider, C. Moser et al., Dual targeting of Raf and VEGF receptor 2 reduces growth and metastasis of pancreatic cancer through direct effects on tumor cells, endothelial cells, and pericytes. Mol. Cancer Ther. 7, 3509 (2008). https://doi.org/10.1158/1535-7163.mct-08-0373
J. Tan, P. Pickhardt, Y. Gao et al., 3D-GLCM CNN: a 3-dimensional gray-level co-occurrence matrix-based CNN model for polyp classification via CT colonography. IEEE T. Med. Imaging. 39, 2013 (2019). https://doi.org/10.1109/TMI.2019.2963177
G.S. Hong, J.C. Lee, J.T. Robinson et al., Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nat. Med. 18, 1841 (2012). https://doi.org/10.1038/nm.2995
Acknowledgements
We thank the staff from the BL13HB beamline of the X-ray Imaging and Biomedical Center, BL08U1-A beamline of the Soft X-ray Spectro-Microscopy Center, BL06B beamline of the Dynamic Beamline: IR Branch at the Shanghai Synchrotron Radiation Facility, and BL01B beamline of the National Facility for Protein Science in Shanghai for their assistance with data collection.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Gong-Xiang Wei, Sui-Xia Zhang, Fu-Li Wang, Zhao Li, Yan-Ling Xue, and Te Ji. The first draft of the manuscript was written by Gong-Xiang Wei and Hui-Qiang Liu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was supported by the Natural Science Foundation of Shandong Province, China (No. ZR2020MA088), Natural Science Foundation of Xinjiang Uygur Autonomous Region, China (No. 2019D01C188), National Key Research and Development Program of China (No. 2018YFC1200204), and National Natural Science Foundation of China (No. 12175127).
Rights and permissions
About this article
Cite this article
Wei, GX., Zhang, SX., Li, Z. et al. Multi-modality measurement and comprehensive analysis of hepatocellular carcinoma using synchrotron-based microscopy and spectroscopy. NUCL SCI TECH 32, 102 (2021). https://doi.org/10.1007/s41365-021-00927-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41365-021-00927-6