Abstract
The Zn–Al–Cl Layered Double Hydroxide (Zn–Al–Cl LDH) was synthesized at constant pH by coprecipitation method and then used as an adsorbent for the removal of a reactive azo dye Remazol Red 23 (RR-23). The structural and morphological properties of the synthesized Zn–Al–Cl LDH were determined using X-ray diffraction (XRD), Fourier Transform Infrared spectroscopy (FTIR), Brunauer–Emmett–Teller surface area, Scanning Electron Microscopy, Energy Dispersive X-ray Spectroscopy, Thermogravimetry Analysis, and Differential Thermal Analysis, and the determination of the point of zero charge pHpzc. The adsorption characteristics were studied by optimizing different parameters such as solution pH, contact time, and initial dye concentration. The maximum adsorption capacity was obtained in the pH ranging from 4 to 7. XRD and FTIR results of Zn–Al–Cl LDH before and after adsorption showed that the removal of the dye is due to its adsorption on the surface of the LDH and that there is no intercalation of the dye since no change in the basal spacing was observed. The kinetic data obtained were examined using the pseudo-first and pseudo-second-order equations. The equilibrium adsorption data were studied using the Freundlich and Langmuir models. The adsorption behavior of Zn–Al–Cl LDH fits perfectly the Langmuir isotherm and the pseudo second-order kinetic model. Thermodynamic studies revealed that the RR-23 dye adsorption on Zn–Al–Cl LDH was exothermic and governed by a physisorption \((\Delta_{{{\text{ads}}}} H^{0} < 0)\), spontaneous \((\Delta_{{{\text{ads}}}} G^{0} < 0)\) and increased the randomness \((\Delta_{{{\text{ads}}}} S^{0} > 0)\) in the adsorbent/adsorbate system. The regeneration study was also conducted in three cycles with 98, 91, and 74% removal efficiency of RR-23.
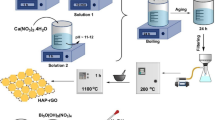
Graphical abstract



















Similar content being viewed by others
References
de Sousa ML, de Moraes PB, Lopes PRM et al (2012) Contamination by Remazol red brilliant dye and its impact in aquatic photosynthetic microbiota. Environ Manag Sustain Dev 1:129–138. https://doi.org/10.5296/emsd.v1i2.2512
Peláez A, Herrera-González AM, Salazar-Villanueva M, Bautista-Hernández A (2016) Elimination of textile dyes using activated carbons prepared from vegetable residues and their characterization. J Environ Manag 181:269–278. https://doi.org/10.1016/j.jenvman.2016.06.026
Tüfekci N, Sivri N, Toroz İ (2007) Pollutants of textile industry wastewater and assessment of its discharge limits by water quality standards. Turk J Fish Aquat Sci 7:97–103
Nasar S (2018) Elimination of a carcinogenic anionic dye Congo red from water using hydrogels based on Chitosan. Acryl Graph Oxide J Bioprocess Biotech 8:1000334
Rezaee A, Ghaneian M, Hashemian S et al (2008) Decolorization of reactive blue 19 dye from textile wastewater by the UV/H2O2 process. J Appl Sci 8:1108–1112. https://doi.org/10.3923/jas.2008.1108.1112
Arab L, Boutahala M, Djellouli B (2014) Étude de l’élimination du Cr(VI) par l’oxyde mixte obtenu par calcination de l’hydroxyde double lamellaire MgAl. Comptes Rendus Chim 17:860–868. https://doi.org/10.1016/j.crci.2014.01.013
Liang X, Zang Y, Xu Y et al (2013) Sorption of metal cations on layered double hydroxides. Colloids Surf Physicochem Eng Asp 433:122–131. https://doi.org/10.1016/j.colsurfa.2013.05.006
Johnston A-L, Lester E, Williams O, Gomes RL (2021) Understanding layered double hydroxide properties as sorbent materials for removing organic pollutants from environmental waters. J Environ Chem Eng 9:105197. https://doi.org/10.1016/j.jece.2021.105197
Zhang B, Huang K, Wang Q et al (2020) Highly efficient treatment of oily wastewater using magnetic carbon nanotubes/layered double hydroxides composites. Colloids Surf Physicochem Eng Asp 586:124187. https://doi.org/10.1016/j.colsurfa.2019.124187
Benício LP, Silva R, Lopes J et al (2015) Layered double hydroxides: nanomaterials for applications in agriculture. Rev Bras Ciênc Solo 39:1–13. https://doi.org/10.1590/01000683rbcs20150817
Cruz IF, Freire C, Araújo JP et al (2018) Chapter 3-multifunctional ferrite nanoparticles: from current trends toward the future. In: El-Gendy AA, Barandiarán JM, Hadimani RL (eds) Magnetic nanostructured materials. Elsevier, New York, pp 59–116
Sato T, Fujita H, Endo T, et al (1988) Synthesis of hydrotalcite-like compounds and their physico-chemical properties (undefined)
Taylor RM, Schwertmann U, Fechter H (1985) A rapid method for the formation of Fe(II), Fe(III) hydroxycarbonate. Clay Miner 20:147–151. https://doi.org/10.1180/claymin.1985.020.1.11
Miyata S (1975) The syntheses of hydrotalcite-like compounds and their structures and physico-chemical properties—I: the systems Mg2+-Al3+-NO3−, Mg2+-Al3+-Cl−, Mg2+-Al3+-ClO4−, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−. Clays Clay Miner 23:369–375. https://doi.org/10.1346/CCMN.1975.0230508
Legrouri A, Badreddine M, Barroug A et al (1999) Influence of pH on the synthesis of the Zn–Al–nitrate layered double hydroxide and the exchange of nitrate by phosphate ions. J Mater Sci Lett 18:1077–1079. https://doi.org/10.1023/A:1006647505203
Lafi R, Charradi K, Djebbi MA et al (2016) Adsorption study of Congo red dye from aqueous solution to Mg–Al–layered double hydroxide. Adv Powder Technol 27:232–237. https://doi.org/10.1016/j.apt.2015.12.004
Djebbi MA, Bouaziz Z, Elabed A et al (2016) Preparation and optimization of a drug delivery system based on berberine chloride-immobilized MgAl hydrotalcite. Int J Pharm 506:438–448. https://doi.org/10.1016/j.ijpharm.2016.04.048
Wang Q, Gao Y, Luo J et al (2013) Synthesis of nano-sized spherical Mg3Al–CO3 layered double hydroxide as a high-temperature CO2 adsorbent. RSC Adv 3:3414–3420. https://doi.org/10.1039/C2RA22607C
Šljivić-Ivanović M, Smičiklas I (2020) 23-Utilization of C&D waste in radioactive waste treatment—current knowledge and perspectives. In: Pacheco-Torgal F, Ding Y, Colangelo F et al (eds) Advances in construction and demolition waste recycling. Woodhead Publishing, New York, pp 475–500
Taoufik N, Boumya W, Elhalil A, et al (2021) Gallic acid removal using fresh and calcined Ni–Al layered double hydroxides: kinetics, equilibrium and response surface methodology (RSM) optimisation
Pechenyuk SI (1999) The use of the pH at the point of zero charge for characterizing the properties of oxide hydroxides. Russ Chem Bull 48:1017–1023. https://doi.org/10.1007/BF02495994
Noh JS, Schwarz JA (1989) Estimation of the point of zero charge of simple oxides by mass titration. J Colloid Interface Sci 130:157–164. https://doi.org/10.1016/0021-9797(89)90086-6
Ouasfi N, Zbair M, Sabbar E, Khamliche L (2019) High Performance of Zn–Al–CO3 Layered double hydroxide for anionic reactive blue 21 dye adsorption: kinetic, equilibrium, and thermodynamic studies. Nanotechnol Environ Eng. https://doi.org/10.1007/s41204-019-0063-5
Daud M, Hai A, Banat F et al (2019) A review on the recent advances, challenges and future aspect of layered double hydroxides (LDH)—containing hybrids as promising adsorbents for dyes removal. J Mol Liq 288:110989. https://doi.org/10.1016/j.molliq.2019.110989
Ai L, Zhang C, Meng L (2011) Adsorption of methyl orange from aqueous solution on hydrothermal synthesized Mg–Al layered double hydroxide. J Chem Eng Data 56:4217–4225. https://doi.org/10.1021/je200743u
Cestari AR, Vieira EFS, Vieira GS, Almeida LE (2006) The removal of anionic dyes from aqueous solutions in the presence of anionic surfactant using aminopropylsilica—a kinetic study. J Hazard Mater 138:133–141. https://doi.org/10.1016/j.jhazmat.2006.05.046
Borhan A, Yusup S, Lim JW, Show PL (2019) Characterization and modelling studies of activated carbon produced from rubber-seed shell using KOH for CO2 Adsorption. Processes 7:855. https://doi.org/10.3390/pr7110855
Ho YS, Mckay G (1998) The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Can J Chem Eng 76:822–827. https://doi.org/10.1002/cjce.5450760419
Elmorsi TM (2011) Equilibrium isotherms and kinetic studies of removal of methylene blue dye by adsorption onto Miswak leaves as a natural adsorbent. J Environ Prot 2:817–827. https://doi.org/10.4236/jep.2011.26093
Ayawei N, Ebelegi AN, Wankasi D (2017) Modelling and Interpretation of Adsorption Isotherms. J Chem 2017:e3039817. https://doi.org/10.1155/2017/3039817
Dąbrowski A (2001) Adsorption—from theory to practice. Adv Colloid Interface Sci 93:135–224. https://doi.org/10.1016/S0001-8686(00)00082-8
Ayawei N, Angaye SS, Wankasi D, Dikio ED (2015) Synthesis, characterization and application of Mg/Al layered double hydroxide for the degradation of Congo red in aqueous solution. Open J Phys Chem 5:56–70. https://doi.org/10.4236/ojpc.2015.53007
Nimibofa A, Ekubo A, Donbebe W, Dikio E (2015) Adsorption of Congo Red by Ni/Al-CO3: equilibrium, thermodynamic and kinetic studies. Orient J Chem 31:1307–1318. https://doi.org/10.13005/ojc/310307
Boparai HK, Joseph M, O’Carroll DM (2011) Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J Hazard Mater 186:458–465. https://doi.org/10.1016/j.jhazmat.2010.11.029
Costa JAS, Paranhos CM (2019) Evaluation of rice husk ash in adsorption of Remazol Red dye from aqueous media. SN Appl Sci 1:397. https://doi.org/10.1007/s42452-019-0436-1
Fettouche S, Tahiri M, Madhouni R, Cherkaoui O (2015) Removal of reactive dyes from aqueous solution by adsorption onto Alfa fibers powder. J Mater Environ Sci 6:129–137
Benhiti R, Ait Ichou A, Zaghloul A et al (2021) Kinetic, isotherm, thermodynamic and mechanism investigations of dihydrogen phosphate removal by MgAl-LDH. Nanotechnol Environ Eng 6:16. https://doi.org/10.1007/s41204-021-00110-7
Lima EC, Hosseini-Bandegharaei A, Moreno-Piraján JC, Anastopoulos I (2019) A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J Mol Liq 273:425–434. https://doi.org/10.1016/j.molliq.2018.10.048
Aarfane A, Salhi A, Krati ME, et al (2014) Etude cinétique et thermodynamique de l’adsorption des colorants Red195 et Bleu de méthylène en milieu aqueux sur les cendres volantes et les mâchefers (Kinetic and thermodynamic study of the adsorption of Red195 and Methylene blue dyes on fly ash and bottom ash in aqueous medium). 13
Boubakri S, Djebbi MA, Bouaziz Z et al (2018) Removal of two anionic reactive textile dyes by adsorption into MgAl-layered double hydroxide in aqueous solutions. Environ Sci Pollut Res 25:23817–23832. https://doi.org/10.1007/s11356-018-2391-6
Khalili R, Ghaedi M, Parvinnia M, Sabzehmeidani MM (2021) Simultaneous removal of binary mixture dyes using mn–Fe layered double hydroxide coated chitosan fibers prepared by wet spinning. Surf Interfaces 23:100976. https://doi.org/10.1016/j.surfin.2021.100976
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tajat, N., El Hayaoui, W., Bougdour, N. et al. Utilization of Zn–Al–Cl layered double hydroxide as an adsorbent for the removal of anionic dye Remazol Red 23 in aqueous solutions: kinetic, equilibrium, and thermodynamic studies. Nanotechnol. Environ. Eng. 7, 343–357 (2022). https://doi.org/10.1007/s41204-022-00237-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41204-022-00237-1