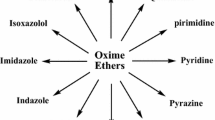
Abstract
Oxime esters as the applicable building blocks, internal oxidizing agents, and directing groups in the synthesis of –, S-, and O-containing heterocycle scaffolds have gained great attention in the last decade. This review provides an overview of recent advances in the cyclization of oxime esters with various functional group reagents under transition metal and transition metal-free catalyzed conditions. Moreover, the mechanistic aspects of these protocols are explained in detail.
Graphical Abstract






















































Similar content being viewed by others
Abbreviations
- SET:
-
Single electron transfer
- DMSO:
-
Dimethyl sulfoxide
- DBU:
-
1,8-Diazabicyclo[5.4.0]undec-7-ene
- DMA:
-
Dimethylacetamide
- DCE:
-
1,2-Dichloroethane
- TEMPO:
-
(2,2,6,6-Tetramethylpiperidin-1-yl)oxyl
- DMF:
-
Dimethylformamide
- DCM:
-
Dichloroethane
- EWG:
-
Electron-withdrawing group
- TEA:
-
Triethylamine
- IMPDH:
-
Inosine-5′-monophosphatase dehydrogenase
- Py:
-
Pyridine
- NMP:
-
N-methyl-2-pyrrolidone
- EDC:
-
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide
- PivOH:
-
Pivalic acid
- THF:
-
Tetrahydrofuran
- TBD:
-
Triazabicyclodecene
- TM:
-
Transition metal
- SCE:
-
Saturated calomel electrode
References
Kundu SK, Rahman M, Dhara P, Hajra A, Majee A (2012) Efficient and alternative approach for preparation of O-benzoyloximes using benzoyl peroxide. Synth Commun 42:1848–1854. https://doi.org/10.1080/00397911.2010.545165
Santosh Kumar SC, Vijendra Kumar N, Srinivas P, Bettadaiah BK (2014) A convenient practical synthesis of alkyl and aryl oxime esters. Synthesis 46:1847–1852. https://doi.org/10.1055/s-0034-1378350
Dikusar EA, Zhukovskaya NA (2008) Preparative synthesis of cyclohexanone oxime esters. Russ J Org Chem 44:1389–1391. https://doi.org/10.1134/S1070428008090248
Zhukovskaya NA, Dikusar EA, Moiseichuk KL, Vyglazov OG (2006) Preparative synthesis of menthone oxime esters. Russ J Appl Chem 79:634–636. https://doi.org/10.1134/S1070427206040252
March J (2007) March’s advanced organic chemistry: reactions, mechanisms and structure. Wiley, New York, pp 513–514
Huang H, Cai J, Deng GJ (2016) O-Acyl oximes: versatile building blocks for N-heterocycle formation in recent transition metal catalysis. Org Biomol Chem 14:1519–1530. https://doi.org/10.1039/c5ob02417j
Tang X, Huang L, Qi C, Wu W, Jiang H (2013) An efficient synthesis of polysubstituted pyrroles via copper-catalyzed coupling of oxime acetates with dialkyl acetylenedicarboxylates under aerobic conditions. Chem Commun 49:9597–9599. https://doi.org/10.1039/c3cc44896g
Senadi GC, Lu TY, Dhandabani GK, Wang JJ (2017) Palladium-catalyzed double-isocyanide insertion via oxidative N–O cleavage of acetyl oximes: syntheses of 2H-pyrrol-2-imines. Org Lett 19:1172–1175. https://doi.org/10.1021/acs.orglett.7b00208
Miao CB, Zheng AQ, Zhou LJ, Lyu X, Yang HT (2020) Copper-catalyzed annulation of oxime acetates with α-amino acid ester derivatives: synthesis of 3-sulfonamido/imino 4-pyrrolin-2-ones. Org Lett 22:3381–3385. https://doi.org/10.1021/acs.orglett.0c00870
Wang ZH et al (2022) Copper-catalyzed ring-opening (3+2) annulation of cyclopropenones with ketoxime acetates: access to 1,2-dihydro-pyrrol-3-ones bearing a quaternary carbon center. Eur J Org Chem. https://doi.org/10.1002/ejoc.202200110
Jones DG et al (2005) Discovery of non-steroidal mifepristone mimetics: pyrazoline-based PR antagonists. Bioorg Med Chem Lett 15:3203–3206. https://doi.org/10.1016/j.bmcl.2005.05.001
Minu M, Thangadurai A, Wakode SR, Agrawal SS, Narasimhan B (2009) Synthesis, antimicrobial activity and QSAR studies of new 2,3-disubstituted-3,3a,4,5,6,7-hexahydro-2H-indazoles. Bioorg Med Chem Lett 19:2960–2964. https://doi.org/10.1016/j.bmcl.2009.04.052
Sahoo A, Yabanoglu S, Sinha BN, Ucar G, Basu A, Jayaprakash V (2010) Towards development of selective and reversible pyrazoline based MAO-inhibitors: synthesis, biological evaluation and docking studies. Bioorg Med Chem Lett 20:132–136. https://doi.org/10.1016/j.bmcl.2009.11.015
Wu Q, Zhang Y, Cui S (2014) Divergent syntheses of 2-aminonicotinonitriles and pyrazolines by copper-catalyzed cyclization of oxime ester. Org Lett 16:1350–1353. https://doi.org/10.1021/ol500094w
DeWald A, Lobbestael S, Poschel BP (1981) Pyrazolodiazepines. 3.4-Aryl- 1,6,7,8- tetrahydro-1,3-dialkylpyrazolo[3,4-e ] [1,4]diazepines as antidepressant agents. J Med Chem 24:982–987
Penning TD et al (1997) Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)- 3(trifluoromethyl)-1h-pyrazol-1-yl]benzenesulfonamide (sc-58635, celecoxib). J Med Chem 40:1347–1365. https://doi.org/10.1021/jm960803q
Terrett NK, Bell AS, Brown D, Ellis P (1996) Sildenafil (Viagra(TM)), a potent and selective inhibitor of type 5 CGMP phosphodiesterase with utility for the treatment of male erectile dysfunction. Bioorg Med Chem Lett 6:1819–1824. https://doi.org/10.1016/0960-894X(96)00323-X
Xiaodong Tang HJ, Huang L, Yang J, Xu Y, Wu W (2014) Practical synthesis of pyrazoles via copper-catalyzed relay oxidation strategy. Chem Commun 50:14793–14796. https://doi.org/10.1039/b000000x
Yadav A et al (2022) Copper-catalyzed oxidative [3+2]-annulation of quinoxalin-2 (1 H )-one with oxime esters toward functionalized pyrazolo [1, 5-a] quinoxalin-4 (5 H)-ones as opioid receptor modulators. J Org Chem 87:7350–7364. https://doi.org/10.1021/acs.joc.2c00563
Zhu Z et al (2017) Iron-catalyzed synthesis of 2H-imidazoles from oxime acetates and vinyl azides under redox-neutral conditions. Org Lett 19:1370–1373. https://doi.org/10.1021/acs.orglett.7b00203
Sun S, Huang J, Yuan C, Wang G, Guo D, Wang J (2022) Switchable assembly of substituted pyrimidines and 2H-imidazoles via Cu(I)-catalysed ring expansion of 2-meoxyl-2H-azirines. Org Chem Front. https://doi.org/10.1039/d2qo00341d
Zhu C et al (2018) Copper-catalyzed coupling of oxime acetates and aryldiazonium salts: an azide-free strategy toward: N-2-aryl-1,2,3-triazoles. Org Chem Front 5:571–576. https://doi.org/10.1039/c7qo00874k
Huang H, Cai J, Ji X, Xiao F, Chen Y, Deng GJ (2016) Internal oxidant-triggered aerobic oxygenation and cyclization of indoles under copper catalysis. Angew Chem Int Ed 55:307–311. https://doi.org/10.1002/anie.201508076
Qu Z, Zhang F, Deng GJ, Huang H (2019) Regioselectivity control in the oxidative formal [3 + 2] annulations of ketoxime acetates and tetrohydroisoquinolines. Org Lett 21:8239–8243. https://doi.org/10.1021/acs.orglett.9b02978
Upare A, Chouhan NK, Ramaraju A, Sridhar B, Bathula SR (2020) Access to pyrrolo[2,1-: A] isoindolediones from oxime acetates and ninhydrin via Cu(I)-mediated domino annulations. Org Biomol Chem 18:1743–1746. https://doi.org/10.1039/d0ob00058b
Huang H, Ji X, Tang X, Zhang M, Li X, Jiang H (2013) Conversion of pyridine to imidazo[1,2-a]pyridines by copper-catalyzed aerobic dehydrogenative cyclization with oxime esters. Org Lett 15:6254–6257. https://doi.org/10.1021/ol403105p
Duan J, Cheng Y, Li R, Li P (2016) Synthesis of spiro[indane-1,3-dione-1-pyrrolines]: via copper-catalyzed heteroannulation of ketoxime acetates with 2-arylideneindane-1,3-diones. Org Chem Front 3:1614–1618. https://doi.org/10.1039/c6qo00454g
Zhao B, Liang HW, Yang J, Yang Z, Wei Y (2017) Copper-catalyzed intermolecular cyclization between oximes and alkenes: a facile access to spiropyrrolines. ACS Catal 7:5612–5617. https://doi.org/10.1021/acscatal.7b01876
Gulati U, Rajesh UC, Rawat DS (2020) Magnetically recoverable Ni@CuI hybrid nanocatalysts affording spiropyrroline heterocycles from ketoximes and alkenes. Asian J Org Chem 9:1059–1064. https://doi.org/10.1002/ajoc.202000145
Ahmad G et al (2006) Synthesis of novel benzofuran isoxazolines as protein tyrosine phosphatase 1B inhibitors. Bioorg Med Chem Lett 16:2139–2143. https://doi.org/10.1016/j.bmcl.2006.01.062
Huang H, Li F, Xu Z, Cai J, Ji X, Deng GJ (2017) Base-promoted [3+2]-annulation of oxime esters and aldehydes for rapid isoxazoline formation. Adv Synth Catal 359:3102–3107. https://doi.org/10.1002/adsc.201700730
Tang X, Zhu Z, Qi C, Wu W, Jiang H (2016) Copper-catalyzed coupling of oxime acetates with isothiocyanates: a strategy for 2-aminothiazoles. Org Lett 18:180–183. https://doi.org/10.1021/acs.orglett.5b03188
Zhu Z, Tang X, Cen J, Li J, Wu W, Jiang H (2018) Copper-catalyzed synthesis of thiazol-2-yl ethers from oxime acetates and xanthates under redox-neutral conditions. Chem Commun 54:3767–3770. https://doi.org/10.1039/c8cc00445e
Zhao L, Brinton RD (2005) Structure-based virtual screening for plant-based ER-selective ligands as potential preventative therapy against age-related neurodegenerative diseases. J Med Chem 48:3463–3466
Wang X, Nakagawa-goto K, Kozuka M, Tokuda H, Nishino H (2006) Cancer preventive agents. Part 6: chemopreventive potential of furanocoumarins and related compounds. Pharm Biol 44:116–120. https://doi.org/10.1080/13880200600592178
Bansal Y, Sethi P, Bansal G (2012) Coumarin: a potential nucleus for anti-inflammatory molecules. Med Chem Res 22:3049–3060. https://doi.org/10.1007/s00044-012-0321-6
Medina FG, Marrero G, Mac M, Garc AGT (2015) Coumarin heterocyclic derivatives: chemical synthesis and biological activity. Nat Prod Rep. https://doi.org/10.1039/C4NP00162A
He M, Yan Z, Wang W, Zhu F, Lin S (2018) Copper-catalyzed radical/radical cross-coupling of ketoxime carboxylates with 4-hydroxycoumarins: a novel synthesis of furo[3,2-c]-coumarins. Tetrahedron Lett 59:3706–3712. https://doi.org/10.1016/j.tetlet.2018.09.007
Pham QT et al (2020) Iodine-mediated formal [3 + 2] annulation for synthesis of furocoumarin from oxime esters. RSC Adv 10:44332–44338. https://doi.org/10.1039/d0ra07566c
Jiang H, Yang J, Tang X, Li J, Wu W (2015) Cu-catalyzed three-component cascade annulation reaction: an entry to functionalized pyridines. J Org Chem 80:8763–8771. https://doi.org/10.1021/acs.joc.5b01621
Zhao MN, Ren ZH, Yu L, Wang YY, Guan ZH (2016) Iron-catalyzed cyclization of ketoxime carboxylates and tertiary anilines for the synthesis of pyridines. Org Lett 18:1194–1197. https://doi.org/10.1021/acs.orglett.6b00326
Gao Q, Yan H, Wu M, Sun J, Yan X, Wu A (2018) Direct synthesis of 2-methylpyridines via I2-triggered [3 + 2 + 1] annulation of aryl methyl ketoxime acetates with triethylamine as the carbon source. Org Biomol Chem 16:2342–2348. https://doi.org/10.1039/c8ob00349a
Yi Y, Zhao MN, Ren ZH, Wang YY, Guan ZH (2017) Synthesis of symmetrical pyridines by iron-catalyzed cyclization of ketoxime acetates and aldehydes. Green Chem 19:1023–1027. https://doi.org/10.1039/c6gc03137d
Beevers RE et al (2006) Novel indole inhibitors of IMPDH from fragments: synthesis and initial structure–activity relationships. Bioorg Med Chem Lett 16:2539–2542. https://doi.org/10.1016/j.bmcl.2006.01.090
Gao Q, Wang Y, Wang Q, Zhu Y, Liu Z, Zhang J (2018) I2-Triggered N–O cleavage of ketoxime acetates for the synthesis of 3-(4-pyridyl)indoles. Org Biomol Chem 16:9030–9037. https://doi.org/10.1039/c8ob02230e
Guo X, Yang X, Qin M, Liu Y, Yang Y, Chen B (2018) Copper-catalyzed cyclization of ketoxime carboxylates and N-aryl glycine esters for the synthesis of pyridines. Asian J Org Chem 7:692–696. https://doi.org/10.1002/ajoc.201700703
Upare A, Sathyanarayana P, Kore R, Sharma K, Bathula SR (2018) Catalyst free synthesis of mono- and disubstituted pyrimidines from O-acyl oximes. Tetrahedron Lett 59:2430–2433. https://doi.org/10.1016/j.tetlet.2018.05.023
Wang Z, Shen L, Yang P, You Y, Zhao J, Yuan W (2022) Access to 4-tri fluoromethyl quinolines via Cu-catalyzed annulation reaction of ketone oxime acetates with ortho-tri fluoroacetyl anilines under redox-neutral conditions. J Org Chem 87:5804–5816. https://doi.org/10.1021/acs.joc.2c00128
Wang Q, Lou J, Huang Z, Yu Z (2019) Rhodium(III)-catalyzed annulation of acetophenone O-acetyl oximes with allenoates through arene C–H activation: an access to isoquinolines. J Org Chem 84:2083–2092. https://doi.org/10.1021/acs.joc.8b03092
Yang X, Liu S, Yu S, Kong L, Lan Y, Li X (2018) Redox-neutral access to isoquinolinones via rhodium(III)-catalyzed annulations of O-pivaloyl oximes with ketenes. Org Lett 20:2698–2701. https://doi.org/10.1021/acs.orglett.8b00906
Jiang H, Yang J, Tang X, Wu W (2016) Divergent syntheses of isoquinolines and indolo[1,2-a]quinazolines by copper-catalyzed cascade annulation from 2-haloaryloxime acetates with active methylene compounds and indoles. J Org Chem 81:2053–2061. https://doi.org/10.1021/acs.joc.5b02914
Zhang ZW, Lin A, Yang J (2014) Methyl ketone oxime esters as nucleophilic coupling partners in Pd-catalyzed C–H alkylation and application in the synthesis of isoquinolines. J Org Chem 79:7041–7050. https://doi.org/10.1021/jo5010586
Yang J et al (2018) Construction of benzene rings by copper-catalyzed cycloaddition reactions of oximes and maleimides: an access to fused phthalimides. Org Lett 20:1216–1219. https://doi.org/10.1021/acs.orglett.8b00141
Yang W et al (2018) Rhodium(III)-catalyzed three-component cascade synthesis of 6H-benzo[c]chromenes through C–H activation. Org Biomol Chem 16:6865–6869. https://doi.org/10.1039/C8OB01938J
Yang W, Zhang H et al (2022) Rh(III)-catalyzed synthesis of dibenzo[b, d]pyran-6-ones from aryl ketone O-acetyl oximes and quinones via C–H activation and C–C bond cleavage. RSC Adv 12:14435–14448. https://doi.org/10.1039/d2ra02074b
Huang H, Xu Z, Ji X, Li B, Deng GJ (2018) Thiophene-fused heteroaromatic systems enabled by internal oxidant-induced cascade Bis-heteroannulation. Org Lett 20:4917–4920. https://doi.org/10.1021/acs.orglett.8b02049
Huang H, Wang Q, Xu Z, Deng GJ (2019) Tri-Functional elemental sulfur enabling bis-heteroannulation of methyl ketoximes with methyl N-heteroarenes. Adv Synth Catal 361:591–596. https://doi.org/10.1002/adsc.201801324
Pham PH, Nguyen KX, Nguyen NP, Pham HTB, Nguyen TT, Phan NTS (2020) 2-Benzoyl thienothiazoles from annulation of C−H bonds in acetophenone oximes. Asian J Org Chem 9:622–625. https://doi.org/10.1002/ajoc.202000046
Zhou P et al (2019) Direct access to bis-S-heterocycles via copper-catalyzed three component tandem cyclization using S 8 as a sulfur source. Org Biomol Chem 17:3424–3432. https://doi.org/10.1039/c9ob00377k
Huang H, Qu Z, Ji X, Deng GJ (2019) Three-component bis-heterocycliation for synthesis of 2-aminobenzo[4,5]thieno[3,2-: D] thiazoles. Org Chem Front 6:1146–1150. https://doi.org/10.1039/c8qo01365a
Zhao J, Bao Y, Wang Y et al (2019) Copper-catalyzed group transfer radical cyclization of γ,δ-unsaturated oxime esters: synthesis of ester functionalized pyrrolines. Asian J Org Chem 8:1317–1320. https://doi.org/10.1002/ajoc.201900399
Wang Y, Ding J, Zhao J, Sun W, Lian C, Chen C (2019) Iminyl radical-promoted imino sulfonylation, imino cyanogenation and imino thiocyanation of γ,δ-unsaturated oxime esters: synthesis of versatile functionalized pyrrolines. Org Chem Front 6:2240–2244. https://doi.org/10.1039/c9qo00421a
Wang L, Wang C (2019) Copper-catalyzed diamination of oxime ester-tethered unactivated alkenes with unprotected amines. J Org Chem 84:6547–6556. https://doi.org/10.1021/acs.joc.9b00936
Chen B et al (2021) Transition-metal-free visible light-induced imino-trifluoromethylation of unsaturated oxime esters: a facile access to CF3-tethered pyrrolines. Asian J Org Chem 10:2360–2364. https://doi.org/10.1002/ajoc.202100429
Cai SH, Xie JH, Song S, Ye L, Feng C, Loh TP (2016) Visible-light-promoted carboimination of unactivated alkenes for the synthesis of densely functionalized pyrroline derivatives. ACS Catal 6:5571–5574. https://doi.org/10.1021/acscatal.6b01230
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors do not have any conflicts of interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yousefnejad, F., Gholami, F., Larijani, B. et al. Oxime Esters: Flexible Building Blocks for Heterocycle Formation. Top Curr Chem (Z) 381, 17 (2023). https://doi.org/10.1007/s41061-023-00431-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-023-00431-y